High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice
- PMID: 21602908
- PMCID: PMC3084703
- DOI: 10.1371/journal.pone.0018556
High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice
Abstract
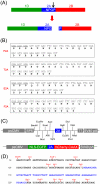
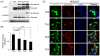
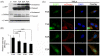
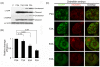
When expression of more than one gene is required in cells, bicistronic or multicistronic expression vectors have been used. Among various strategies employed to construct bicistronic or multicistronic vectors, an internal ribosomal entry site (IRES) has been widely used. Due to the large size and difference in expression levels between genes before and after IRES, however, a new strategy was required to replace IRES. A self-cleaving 2A peptide could be a good candidate to replace IRES because of its small size and high cleavage efficiency between genes upstream and downstream of the 2A peptide. Despite the advantages of the 2A peptides, its use is not widespread because (i) there are no publicly available cloning vectors harboring a 2A peptide gene and (ii) comprehensive comparison of cleavage efficiency among various 2A peptides reported to date has not been performed in different contexts. Here, we generated four expression plasmids each harboring different 2A peptides derived from the foot-and-mouth disease virus, equine rhinitis A virus, Thosea asigna virus and porcine teschovirus-1, respectively, and evaluated their cleavage efficiency in three commonly used human cell lines, zebrafish embryos and adult mice. Western blotting and confocal microscopic analyses revealed that among the four 2As, the one derived from porcine teschovirus-1 (P2A) has the highest cleavage efficiency in all the contexts examined. We anticipate that the 2A-harboring cloning vectors we generated and the highest efficiency of the P2A peptide we demonstrated would help biomedical researchers easily adopt the 2A technology when bicistronic or multicistronic expression is required.
Conflict of interest statement
Figures

References
-
- de Felipe P. Polycistronic viral vectors. Curr Gene Ther. 2002;2:355–378. - PubMed
-
- Szymczak AL, Vignali DA. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin Biol Ther. 2005;5:627–638. - PubMed
-
- de Felipe P, Luke GA, Hughes LE, Gani D, Halpin C, et al. E unum pluribus: multiple proteins from a self-processing polyprotein. Trends Biotechnol. 2006;24:68–75. - PubMed
-
- Ryan MD, King AM, Thomas GP. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol. 1991;72(Pt 11):2727–2732. - PubMed
-
- Lyan Lab Webpage. Available: http://www.st-andrews.ac.uk/ryanlab/Index.htm. Accessed 2011 Mar 15.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

