The Contribution of DOPA to Substrate-Peptide Adhesion and Internal Cohesion of Mussel-Inspired Synthetic Peptide Films
- PMID: 21603098
- PMCID: PMC3098815
- DOI: 10.1002/adfm.201000932
The Contribution of DOPA to Substrate-Peptide Adhesion and Internal Cohesion of Mussel-Inspired Synthetic Peptide Films
Abstract
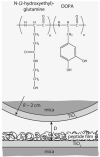
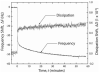
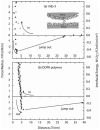
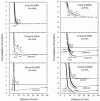
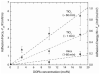
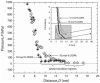
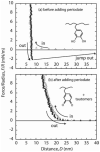
Mussels use a variety of 3, 4-dihydroxyphenyl-l-alanine (DOPA) rich proteins specifically tailored to adhering to wet surfaces. Synthetic polypeptide analogues of adhesive mussel foot proteins (specifically mfp-3) are used to study the role of DOPA in adhesion. The mussel-inspired peptide is a random copolymer of DOPA and N(5) -(2-hydroxyethyl)-l-glutamine synthesized with DOPA concentrations of 0-27 mol% and molecular weights of 5.9-7.1 kDa. Thin films (3-5 nm thick) of the mussel-inspired peptide are used in the surface forces apparatus (SFA) to measure the force-distance profiles and adhesion and cohesion energies of the films in an acetate buffer. The adhesion energies of the mussel-inspired peptide films to mica and TiO(2) surfaces increase with DOPA concentration. The adhesion energy to mica is 0.09 μJ m(-2) mol(DOPA) (-1) and does not depend on contact time or load. The adhesion energy to TiO(2) is 0.29 μJ m(-2) mol(DOPA) (-1) for short contact times and increases to 0.51 μJ m(-2) mol(DOPA) (-1) for contact times >60 min in a way suggestive of a phase transition within the film. Oxidation of DOPA to the quinone form, either by addition of periodate or by increasing the pH, increases the thickness and reduces the cohesion of the films. Adding thiol containing polymers between the oxidized films recovers some of the cohesion strength. Comparison of the mussel-inspired peptide films to previous studies on mfp-3 thin films show that the strong adhesion and cohesion in mfp-3 films can be attributed to DOPA groups favorably oriented within or at the interface of these films.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
