Decreased proliferation kinetics of mouse myoblasts overexpressing FRG1
- PMID: 21603621
- PMCID: PMC3095625
- DOI: 10.1371/journal.pone.0019780
Decreased proliferation kinetics of mouse myoblasts overexpressing FRG1
Abstract
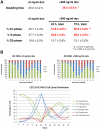
Although recent publications have linked the molecular events driving facioscapulohumeral muscular dystrophy (FSHD) to expression of the double homeobox transcription factor DUX4, overexpression of FRG1 has been proposed as one alternative causal agent as mice overexpressing FRG1 present with muscular dystrophy. Here, we characterize proliferative defects in two independent myoblast lines overexpressing FRG1. Myoblasts isolated from thigh muscle of FRG1 transgenic mice, an affected dystrophic muscle, exhibit delayed proliferation as measured by decreased clone size, whereas myoblasts isolated from the unaffected diaphragm muscle proliferated normally. To confirm the observation that overexpression of FRG1 could impair myoblast proliferation, we examined C2C12 myoblasts with inducible overexpression of FRG1, finding increased doubling time and G1-phase cells in mass culture after induction of FRG1 and decreased levels of pRb phosphorylation. We propose that depressed myoblast proliferation may contribute to the pathology of mice overexpressing FRG1 and may play a part in FSHD.
Conflict of interest statement
Figures



Similar articles
-
FHL1 reduces dystrophy in transgenic mice overexpressing FSHD muscular dystrophy region gene 1 (FRG1).PLoS One. 2015 Feb 19;10(2):e0117665. doi: 10.1371/journal.pone.0117665. eCollection 2015. PLoS One. 2015. PMID: 25695429 Free PMC article.
-
Direct interplay between two candidate genes in FSHD muscular dystrophy.Hum Mol Genet. 2015 Mar 1;24(5):1256-66. doi: 10.1093/hmg/ddu536. Epub 2014 Oct 17. Hum Mol Genet. 2015. PMID: 25326393 Free PMC article.
-
Rbfox1 downregulation and altered calpain 3 splicing by FRG1 in a mouse model of Facioscapulohumeral muscular dystrophy (FSHD).PLoS Genet. 2013;9(1):e1003186. doi: 10.1371/journal.pgen.1003186. Epub 2013 Jan 3. PLoS Genet. 2013. PMID: 23300487 Free PMC article.
-
Deciphering transcription dysregulation in FSH muscular dystrophy.J Hum Genet. 2012 Aug;57(8):477-84. doi: 10.1038/jhg.2012.74. Epub 2012 Jun 21. J Hum Genet. 2012. PMID: 22718021 Free PMC article. Review.
-
Current status and future prospect of FSHD region gene 1.J Biosci. 2017 Jun;42(2):345-353. doi: 10.1007/s12038-017-9681-x. J Biosci. 2017. PMID: 28569257 Review.
Cited by
-
A muscle stem cell for every muscle: variability of satellite cell biology among different muscle groups.Front Aging Neurosci. 2015 Oct 7;7:190. doi: 10.3389/fnagi.2015.00190. eCollection 2015. Front Aging Neurosci. 2015. PMID: 26500547 Free PMC article. Review.
-
Thimerosal-induced apoptosis in mouse C2C12 myoblast cells occurs through suppression of the PI3K/Akt/survivin pathway.PLoS One. 2012;7(11):e49064. doi: 10.1371/journal.pone.0049064. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23145070 Free PMC article.
-
Cellular and animal models for facioscapulohumeral muscular dystrophy.Dis Model Mech. 2020 Oct 28;13(10):dmm046904. doi: 10.1242/dmm.046904. Dis Model Mech. 2020. PMID: 33174531 Free PMC article. Review.
-
Reduced FRG1 expression promotes prostate cancer progression and affects prostate cancer cell migration and invasion.BMC Cancer. 2019 Apr 11;19(1):346. doi: 10.1186/s12885-019-5509-4. BMC Cancer. 2019. PMID: 30975102 Free PMC article.
-
Facioscapulohumeral muscular dystrophy (FSHD): an enigma unravelled?Hum Genet. 2012 Mar;131(3):325-40. doi: 10.1007/s00439-011-1100-z. Epub 2011 Oct 9. Hum Genet. 2012. PMID: 21984394 Review.
References
-
- van der Maarel SM, Frants RR, Padberg GW. Facioscapulohumeral muscular dystrophy. Biochim Biophys Acta. 2007;1772:186–194. - PubMed
-
- Lunt PW, Jardine PE, Koch MC, Maynard J, Osborn M, et al. Correlation between fragment size at D4F104S1 and age at onset or at wheelchair use, with a possible generational effect, accounts for much phenotypic variation in 4q35–facioscapulohumeral muscular dystrophy (FSHD). Hum Mol Genet. 1995;4:951–958. - PubMed
-
- Tawil R, Forrester J, Griggs RC, Mendell J, Kissel J, et al. Evidence for anticipation and association of deletion size with severity in facioscapulohumeral muscular dystrophy. The FSH-DY Group. Ann Neurol. 1996;39:744–748. - PubMed
-
- Ricci E, Galluzzi G, Deidda G, Cacurri S, Colantoni L, et al. Progress in the molecular diagnosis of facioscapulohumeral muscular dystrophy and correlation between the number of KpnI repeats at the 4q35 locus and clinical phenotype. Ann Neurol. 1999;45:751–757. - PubMed
-
- Tawil R, Van Der Maarel SM. Facioscapulohumeral muscular dystrophy. Muscle Nerve. 2006;34:1–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

