Chemopreventive effect of PSP through targeting of prostate cancer stem cell-like population
- PMID: 21603625
- PMCID: PMC3095629
- DOI: 10.1371/journal.pone.0019804
Chemopreventive effect of PSP through targeting of prostate cancer stem cell-like population
Erratum in
- PLoS One. 2011;6(6). doi:10.1371/annotation/0f6309be-936c-4974-97bf-ed3a98289cd9
- PLoS One. 2011;6(6). doi:10.1371/annotation/b0312c4c-e06a-47ef-9e9c-b044dbfa3d6a
Abstract
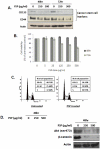
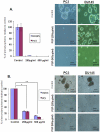
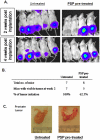
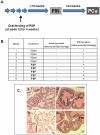
Recent evidence suggested that prostate cancer stem/progenitor cells (CSC) are responsible for cancer initiation as well as disease progression. Unfortunately, conventional therapies are only effective in targeting the more differentiated cancer cells and spare the CSCs. Here, we report that PSP, an active component extracted from the mushroom Turkey tail (also known as Coriolus versicolor), is effective in targeting prostate CSCs. We found that treatment of the prostate cancer cell line PC-3 with PSP led to the down-regulation of CSC markers (CD133 and CD44) in a time and dose-dependent manner. Meanwhile, PSP treatment not only suppressed the ability of PC-3 cells to form prostaspheres under non-adherent culture conditions, but also inhibited their tumorigenicity in vivo, further proving that PSP can suppress prostate CSC properties. To investigate if the anti-CSC effect of PSP may lead to prostate cancer chemoprevention, transgenic mice (TgMAP) that spontaneously develop prostate tumors were orally fed with PSP for 20 weeks. Whereas 100% of the mice that fed with water only developed prostate tumors at the end of experiment, no tumors could be found in any of the mice fed with PSP, suggesting that PSP treatment can completely inhibit prostate tumor formation. Our results not only demonstrated the intriguing anti-CSC effect of PSP, but also revealed, for the first time, the surprising chemopreventive property of oral PSP consumption against prostate cancer.
Conflict of interest statement
Figures

Similar articles
-
Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population.Int J Cancer. 2011 May 1;128(9):2182-91. doi: 10.1002/ijc.25546. Int J Cancer. 2011. PMID: 20617516
-
Using self-assembled nanomaterials to inhibit the formation of metastatic cancer stem cell colonies in vitro.Cell Transplant. 2011;20(1):127-31. doi: 10.3727/096368910X532783. Epub 2010 Sep 30. Cell Transplant. 2011. PMID: 20887677
-
Pristimerin Inhibits Prostate Cancer Bone Metastasis by Targeting PC-3 Stem Cell Characteristics and VEGF-Induced Vasculogenesis of BM-EPCs.Cell Physiol Biochem. 2015;37(1):253-68. doi: 10.1159/000430350. Epub 2015 Aug 20. Cell Physiol Biochem. 2015. PMID: 26302893
-
Targeting prostate cancer stem cells.Anticancer Agents Med Chem. 2009 Dec;9(10):1105-13. doi: 10.2174/187152009789735053. Anticancer Agents Med Chem. 2009. PMID: 19925394 Review.
-
Recent Advances and Challenges in Studies of Control of Cancer Stem Cells and the Gut Microbiome by the Trametes-Derived Polysaccharopeptide PSP (Review).Int J Med Mushrooms. 2016;18(8):651-660. doi: 10.1615/IntJMedMushrooms.v18.i8.10. Int J Med Mushrooms. 2016. PMID: 27910783 Review.
Cited by
-
Adipocytes promote prostate cancer stem cell self-renewal through amplification of the cholecystokinin autocrine loop.Oncotarget. 2016 Jan 26;7(4):4939-48. doi: 10.18632/oncotarget.6643. Oncotarget. 2016. PMID: 26700819 Free PMC article.
-
Transcriptome and proteome exploration to provide a resource for the study of Agrocybe aegerita.PLoS One. 2013;8(2):e56686. doi: 10.1371/journal.pone.0056686. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418592 Free PMC article.
-
Alpha-tomatine attenuation of in vivo growth of subcutaneous and orthotopic xenograft tumors of human prostate carcinoma PC-3 cells is accompanied by inactivation of nuclear factor-kappa B signaling.PLoS One. 2013;8(2):e57708. doi: 10.1371/journal.pone.0057708. Epub 2013 Feb 21. PLoS One. 2013. PMID: 23437404 Free PMC article.
-
Polysaccharide-Peptide from Trametes versicolor: The Potential Medicine for Colorectal Cancer Treatment.Biomedicines. 2022 Nov 7;10(11):2841. doi: 10.3390/biomedicines10112841. Biomedicines. 2022. PMID: 36359361 Free PMC article. Review.
-
In Vivo Immunomodulation and Lipid Peroxidation Activities Contributed to Chemoprevention Effects of Fermented Mung Bean against Breast Cancer.Evid Based Complement Alternat Med. 2013;2013:708464. doi: 10.1155/2013/708464. Epub 2013 Apr 22. Evid Based Complement Alternat Med. 2013. PMID: 23710232 Free PMC article.
References
-
- Pomerantz M, Kantoff P. Advances in the treatment of prostate cancer. Annu Rev Med. 2007;58:205–220. - PubMed
-
- Sarvis JA, Thompson IM. Prostate cancer chemoprevention: update of the prostate cancer prevention trial findings and implications for clinical practice. Curr Oncol Rep. 2008;10:529–532. - PubMed
-
- Musquera M, Fleshner NE, Finelli A, Zlotta AR. The REDUCE trial: chemoprevention in prostate cancer using a dual 5alpha-reductase inhibitor, dutasteride. Expert Rev Anticancer Ther. 2008;8:1073–1079. - PubMed
-
- Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. - PubMed
-
- Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer. 2006;13:751–778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

