Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial
- PMID: 21603629
- PMCID: PMC3094355
- DOI: 10.1371/journal.pone.0019283
Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial
Abstract
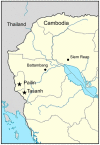
Background: The emergence of artemisinin resistance has raised concerns that the most potent antimalarial drug may be under threat. The currently recommended daily dose of artesunate (AS) is 4 mg/kg, and is administered for 3 days together with a partner antimalarial drug. This study investigated the impact of different AS doses on clinical and parasitological responses in malaria patients from an area of known artemisinin resistance in western Cambodia.
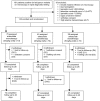
Methods: Adult patients with uncomplicated P. falciparum malaria were randomized into one of three 7-day AS monotherapy regimens: 2, 4 or 6 mg/kg/day (total dose 14, 28 and 42 mg/kg). Clinical, parasitological, pharmacokinetic and in vitro drug sensitivity data was collected over a 7-day inpatient period and during weekly follow-up to 42 days.
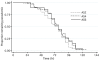
Results: 143 patients were enrolled (n = 75, 40 and 28 to receive AS 2, 4 and 6 mg/kg/day respectively). Cure rates were high in all treatment groups at 42 days despite almost half the patients remaining parasitemic on Day 3. There was no impact of increasing AS dose on median parasite clearance times, median parasite clearance rates or on the proportion of patients remaining parasitemic on Day 3. However at the lowest dose used (2 mg/kg/d) patients with parasitemia >10,000/µL had longer median (IQR) parasite clearance times than those with parasitemia <10,000/µL (63 (48-75) vs. 84 (66-96) hours, p<0.0001). 19% of patients in the high-dose arm developed neutropenia (absolute neutrophil count <1.0×10(9)/L) by Day 14 and resulted in the arm being halted early.
Conclusion: There is no pharmacodynamic benefit of increasing the daily dose of AS (4 mg/kg) currently recommended for short-course combination treatment of uncomplicated malaria, even in regions with emerging artemisinin resistance, as long as the partner drug retains high efficacy.
Trial registration: ClinicalTrials.gov NCT00722150.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

