Use of the 2A peptide for generation of multi-transgenic pigs through a single round of nuclear transfer
- PMID: 21603633
- PMCID: PMC3094386
- DOI: 10.1371/journal.pone.0019986
Use of the 2A peptide for generation of multi-transgenic pigs through a single round of nuclear transfer
Abstract
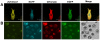
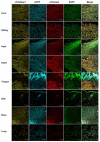
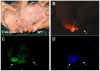
Multiple genetic modifications in pigs can essentially benefit research on agriculture, human disease and xenotransplantation. Most multi-transgenic pigs have been produced by complex and time-consuming breeding programs using multiple single-transgenic pigs. This study explored the feasibility of producing multi-transgenic pigs using the viral 2A peptide in the light of previous research indicating that it can be utilized for multi-gene transfer in gene therapy and somatic cell reprogramming. A 2A peptide-based double-promoter expression vector that mediated the expression of four fluorescent proteins was constructed and transfected into primary porcine fetal fibroblasts. Cell colonies (54.3%) formed under G418 selection co-expressed the four fluorescent proteins at uniformly high levels. The reconstructed embryos, which were obtained by somatic cell nuclear transfer and confirmed to express the four fluorescent proteins evenly, were transplanted into seven recipient gilts. Eleven piglets were delivered by two gilts, and seven of them co-expressed the four fluorescent proteins at equivalently high levels in various tissues. The fluorescence intensities were directly observed at the nose, hoof and tongue using goggles. The results suggest that the strategy of combining the 2A peptide and double promoters efficiently mediates the co-expression of the four fluorescent proteins in pigs and is hence a promising methodology to generate multi-transgenic pigs by a single nuclear transfer.
Conflict of interest statement
Figures
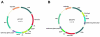



References
-
- Niemann H, Kues WA. Application of transgenesis in livestock for agriculture and biomedicine. Anim Reprod Sci. 2003;79:291–317. - PubMed
-
- Prather RS, Hawley RJ, Carter DB, Lai L, Greenstein JL. Transgenic swine for biomedicine and agriculture. Theriogenology. 2003;59:115–123. - PubMed
-
- Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

