The GPR55 agonist lysophosphatidylinositol directly activates intermediate-conductance Ca2+ -activated K+ channels
- PMID: 21603896
- PMCID: PMC3132407
- DOI: 10.1007/s00424-011-0977-7
The GPR55 agonist lysophosphatidylinositol directly activates intermediate-conductance Ca2+ -activated K+ channels
Abstract
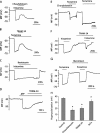
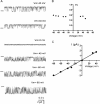
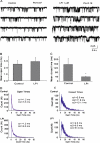
Lysophosphatidylinositol (LPI) was recently shown to act both as an extracellular mediator binding to G protein-coupled receptor 55 (GPR55) and as an intracellular messenger directly affecting a number of ion channels including large-conductance Ca(2+) and voltage-gated potassium (BK(Ca)) channels. Here, we explored the effect of LPI on intermediate-conductance Ca(2+)-activated K(+) (IK(Ca)) channels using excised inside-out patches from endothelial cells. The functional expression of IK(Ca) was confirmed by the charybdotoxin- and TRAM-34-sensitive hyperpolarization to histamine and ATP. Moreover, the presence of single IK(Ca) channels with a slope conductance of 39 pS in symmetric K(+) gradient was directly confirmed in inside-out patches. When cytosolically applied in the range of concentrations of 0.3-10 μM, which are well below the herein determined critical micelle concentration of approximately 30 μM, LPI potentiated the IK(Ca) single-channel activity in a concentration-dependent manner, while single-channel current amplitude was not affected. In the whole-cell configuration, LPI in the pipette was found to facilitate membrane hyperpolarization in response to low (0.5 μM) histamine concentrations in a TRAM-34-sensitive manner. These results demonstrate a so far not-described receptor-independent effect of LPI on the IK(Ca) single-channel activity of endothelial cells, thus, highlighting LPI as a potent intracellular messenger capable of modulating electrical responses in the vasculature.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

