Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4⁺ T cells from patients with GI malignancy
- PMID: 21604071
- PMCID: PMC3521517
- DOI: 10.1007/s00262-011-1029-z
Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4⁺ T cells from patients with GI malignancy
Abstract
Interferon-alpha (IFN-α) promotes anti-tumor immunity through its actions on immune cells. We hypothesized that elevated percentages of myeloid-derived suppressor cells (MDSC) and increased pro-inflammatory cytokines in peripheral blood would be associated with impaired response to IFN-α in patients with gastrointestinal (GI) malignancies. This study evaluated relationships between plasma IL-6, IL-10, circulating MDSC subsets, and IFN-α-induced signal transduction in 40 patients with GI malignancies. Plasma IL-6 and IL-10 were significantly higher in patients versus normal donors. CD33(+)HLADR(-)CD11b(+)CD15(+) and CD33(+)HLADR(-/low)CD14(+) MDSC subsets were also elevated in patients versus normal donors (P < 0.0001). Plasma IL-6 was correlated with CD33(+)HLADR(-)CD15(+) MDSC (P = 0.008) and IL-10 with CD33(+)HLADR(-)CD15(-) MDSC (P = 0.002). The percentage of CD15(+) and CD15(-) but not CD14(+) MDSC subsets were inversely correlated with IFN-α-induced STAT1 phosphorylation in CD4(+) T cells, while co-culture with in vitro generated MDSC led to reduced IFN-α responsiveness in both PBMC and the CD4(+) subset of T cells from normal donors. Exploratory multivariable Cox proportional hazards models revealed that an increased percentage of the CD33(+)HLADR(-)CD15(-) MDSC subset was associated with reduced overall survival (P = 0.049), while an increased percentage of the CD33(+)HLADR(-/low)CD14(+) subset was associated with greater overall survival (P = 0.033). These data provide evidence for a unique relationship between specific cytokines, MDSC subsets, and IFN-α responsiveness in patients with GI malignancies.
Figures

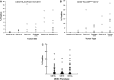

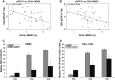
References
-
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

