Developing imaging strategies for castration resistant prostate cancer
- PMID: 21604939
- PMCID: PMC3415271
- DOI: 10.3109/0284186X.2011.572914
Developing imaging strategies for castration resistant prostate cancer
Abstract
Recent advances in the understanding of castrate-resistant prostate cancer (CRPC) have lead to a growing number of experimental therapies, many of which are directed against the androgen-receptor (AR) signaling axis. These advances generate the need for reliable molecular imaging biomarkers to non-invasively determine efficacy, and to better guide treatment selection of these promising AR-targeted drugs. Methods. We draw on our own experience, supplemented by review of the current literature, to discuss the systematic development of imaging biomarkers for use in the context of CRPC, with a focus on bone scintigraphy, F-18 fluorodeoxyglucose (FDG)-positron emission tomography (PET) and PET imaging of the AR signaling axis. Results. The roadmap to biomarker development mandates rigorous standardization and analytic validation of an assay before it can be qualified successfully for use in an appropriate clinical context. The Prostate Cancer Working Group 2 (PCWG2) criteria for "radiographic" progression by bone scintigraphy serve as a paradigm of this process. Implemented by the Prostate Cancer Clinical Trials Consortium (PCCTC), these consensus criteria may ultimately enable the co-development of more potent and versatile molecular imaging biomarkers. Purported to be superior to single-photon bone scanning, the added value of Na(18)F-PET for imaging of bone metastases is still uncertain. FDG-PET already plays an integral role in the management of many diseases, but requires further evaluation before being qualified in the context of CRPC. PET tracers that probe the AR signaling axis, such as (18)F-FDHT and (89)Zr-591, are now under development as pharmacodynamic markers, and as markers of efficacy, in tandem with FDG-PET. Semi-automated analysis programs for facilitating PET interpretation may serve as a valuable tool to help navigate the biomarker roadmap. Conclusions. Molecular imaging strategies, particularly those that probe the AR signaling axis, have the potential to accelerate drug development in CRPC. The development and use of analytically valid imaging biomarkers will increase the likelihood of clinical qualification, and ultimately lead to improved patient outcomes.
Conflict of interest statement
Figures
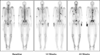





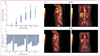
References
-
- Cancer. org [internet]. American Cancer Society. 2011 Available from: http://www.cancer.org/Cancer/ProstateCancer/DetailedGuide/prostate-cance....
-
- Scher H. Prostate carcinoma: Defining therapeutic objectives and improving overall outcomes. Cancer Suppl. 2003;97:758–771. - PubMed
-
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
