Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study
- PMID: 21605396
- PMCID: PMC3146950
- DOI: 10.1186/1471-2466-11-28
Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study
Abstract
Background: The inhaled corticosteroid (ICS) fluticasone propionate (fluticasone) and the long-acting β2-agonist (LABA) formoterol fumarate (formoterol) are being made available as a combination product (fluticasone/formoterol, flutiform ®) in a single aerosol inhaler. This 12-week, open-label, randomized, active-controlled, parallel-group, multicentre, phase 3 study compared the efficacy and safety of fluticasone/formoterol with the commercially available combination product fluticasone/salmeterol.
Methods: Patients aged ≥ 18 years (N = 202) with mild-to-moderate-severe, persistent asthma for ≥ 6 months prior to screening were included in the study. After a screening phase (4-10 days), eligible patients were randomized 1:1 to receive fluticasone/formoterol or fluticasone/salmeterol during the 12-week treatment period. The primary objective was to demonstrate non-inferiority of fluticasone/formoterol versus fluticasone/salmeterol, measured by pre-dose forced expiratory volume in the first second (FEV1), at week 12.
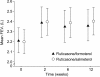
Results: Fluticasone/formoterol was comparable to fluticasone/salmeterol for the primary efficacy endpoint, mean pre-dose FEV1 at week 12. The new combination was also comparable to fluticasone/salmeterol for change from baseline to week 12 in pre-dose FEV1, change from pre-dose FEV1 at baseline to 2-hour post-dose FEV1 at week 12 and discontinuations due to lack of efficacy. Importantly, fluticasone/formoterol was superior to fluticasone/salmeterol in time to onset of action throughout the duration of the study. The two treatments demonstrated similar results for various other secondary efficacy parameters, including other lung function tests, patient-reported outcomes, rescue medication use, asthma exacerbations and Asthma Quality of Life Questionnaire scores. Fluticasone/formoterol was well tolerated and had a good safety profile that was similar to fluticasone/salmeterol.
Conclusions: The results of this study indicate that fluticasone/formoterol is as effective as fluticasone/salmeterol, and has a more rapid onset of action, reflecting the faster bronchodilatory effects of formoterol compared with those of salmeterol. If patients perceive the benefits of therapy with fluticasone/formoterol more rapidly than with fluticasone/salmeterol, this could have a positive impact on preference and adherence.
Trial registration: ClinicalTrials.gov NCT00476073.
Figures

References
-
- Masoli M, Fabian D, Holt S, Beasley R. Global burden of asthma, a report for the Global Initiative for Asthma. 2004. http://www.ginasthma.org - PubMed
-
- Cazzoletti L, Marcon A, Janson C, Corsico A, Jarvis D, Pin I, Accordini S, Almar E, Bugiani M, Carolei A. et al. Asthma control in Europe: a real-world evaluation based on an international population-based study. J Allergy Clin Immunol. 2007;120:1360–1367. doi: 10.1016/j.jaci.2007.09.019. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

