Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants
- PMID: 21605713
- PMCID: PMC3175014
- DOI: 10.1016/j.bbagrm.2011.05.001
Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants
Abstract
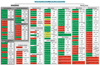
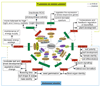
Small, non-coding RNAs are a distinct class of regulatory RNAs in plants and animals that control a variety of biological processes. In plants, several classes of small RNAs with specific sizes and dedicated functions have evolved through a series of pathways. The major classes of small RNAs include microRNAs (miRNAs) and small interfering RNAs (siRNAs), which differ in their biogenesis. miRNAs control the expression of cognate target genes by binding to reverse complementary sequences, resulting in cleavage or translational inhibition of the target RNAs. siRNAs have a similar structure, function, and biogenesis as miRNAs but are derived from long double-stranded RNAs and can often direct DNA methylation at target sequences. Besides their roles in growth and development and maintenance of genome integrity, small RNAs are also important components in plant stress responses. One way in which plants respond to environmental stress is by modifying their gene expression through the activity of small RNAs. Thus, understanding how small RNAs regulate gene expression will enable researchers to explore the role of small RNAs in biotic and abiotic stress responses. This review focuses on the regulatory roles of plant small RNAs in the adaptive response to stresses. This article is part of a Special Issue entitled: Plant gene regulation in response to abiotic stress.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

References
-
- Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. - PubMed
-
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. - PubMed
-
- Hannon GJ. RNA interference. Nature. 2002;418:244–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

