Islet amyloid polypeptide demonstrates a persistent capacity to disrupt membrane integrity
- PMID: 21606325
- PMCID: PMC3111278
- DOI: 10.1073/pnas.1102356108
Islet amyloid polypeptide demonstrates a persistent capacity to disrupt membrane integrity
Abstract
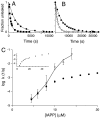
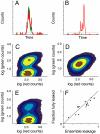
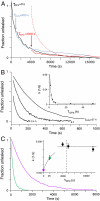
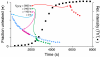
Amyloid fiber formation is correlated with pathology in many diseases, including Alzheimer's, Parkinson's, and type II diabetes. Although β-sheet-rich fibrillar protein deposits define this class of disorder, increasing evidence points toward small oligomeric species as being responsible for cell dysfunction and death. The molecular mechanism by which this occurs is unknown, but likely involves the interaction of these species with biological membranes, with a subsequent loss of integrity. Here, we investigate islet amyloid polypeptide, which is implicated in the loss of insulin-secreting cells in type II diabetics. We report the discovery of oligomeric species that arise through stochastic nucleation on membranes and result in disruption of the lipid bilayer. These species are stable, result in all-or-none leakage, and represent a definable protein/lipid phase that equilibrates over time. We characterize the reaction pathway of assembly through the use of an experimental design that includes both ensemble and single-particle evaluations. Complexity in the reaction pathway could not be satisfied using a two-state description of membrane-bound monomer and oligomeric species. We therefore put forward a three-state kinetic framework, one of which we conjecture represents a non-amyloid, non-β-sheet intermediate previously shown to be a candidate therapeutic target.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Hebda JA, Miranker AD. The interplay of catalysis and toxicity by amyloid intermediates on lipid bilayers: Insights from type II diabetes. Annu Rev Biophys. 2009;38:125–152. - PubMed
-
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
-
- Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006;27:570–575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

