Cholinergic chemosensory cells in the trachea regulate breathing
- PMID: 21606356
- PMCID: PMC3111311
- DOI: 10.1073/pnas.1019418108
Cholinergic chemosensory cells in the trachea regulate breathing
Abstract
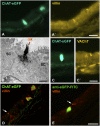
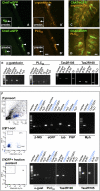
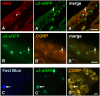
In the epithelium of the lower airways, a cell type of unknown function has been termed "brush cell" because of a distinctive ultrastructural feature, an apical tuft of microvilli. Morphologically similar cells in the nose have been identified as solitary chemosensory cells responding to taste stimuli and triggering trigeminal reflexes. Here we show that brush cells of the mouse trachea express the receptors (Tas2R105, Tas2R108), the downstream signaling molecules (α-gustducin, phospholipase C(β2)) of bitter taste transduction, the synthesis and packaging machinery for acetylcholine, and are addressed by vagal sensory nerve fibers carrying nicotinic acetylcholine receptors. Tracheal application of an nAChR agonist caused a reduction in breathing frequency. Similarly, cycloheximide, a Tas2R108 agonist, evoked a drop in respiratory rate, being sensitive to nicotinic receptor blockade and epithelium removal. This identifies brush cells as cholinergic sensors of the chemical composition of the lower airway luminal microenvironment that are directly linked to the regulation of respiration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sbarbati A, Osculati F. The taste cell-related diffuse chemosensory system. Prog Neurobiol. 2005;75:295–307. - PubMed
-
- Rhodin J, Dalhamn T. Electron microscopy of the tracheal ciliated mucosa in rat. Z Zellforsch Mikrosk Anat. 1956;44:345–412. - PubMed
-
- Sbarbati A, Bramanti P, Benati D, Merigo F. The diffuse chemosensory system: Exploring the iceberg toward the definition of functional roles. Prog Neurobiol. 2010;91:77–89. - PubMed
-
- Merigo F, Benati D, Tizzano M, Osculati F, Sbarbati A. α-Gustducin immunoreactivity in the airways. Cell Tissue Res. 2005;319:211–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

