Hyperglycemic Ins2AkitaLdlr⁻/⁻ mice show severely elevated lipid levels and increased atherosclerosis: a model of type 1 diabetic macrovascular disease
- PMID: 21606463
- PMCID: PMC3137013
- DOI: 10.1194/jlr.M014092
Hyperglycemic Ins2AkitaLdlr⁻/⁻ mice show severely elevated lipid levels and increased atherosclerosis: a model of type 1 diabetic macrovascular disease
Abstract
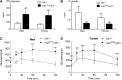
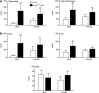
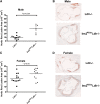
Accelerated atherosclerosis is the leading cause of death in type 1 diabetes, but the mechanism of type 1 diabetes-accelerated atherosclerosis is not well understood, in part due to the lack of a good animal model for the long-term studies required. In an attempt to create a model for studying diabetic macrovascular disease, we have generated type 1 diabetic Akita mice lacking the low density lipoprotein receptor (Ins2(Akita)Ldlr⁻/⁻). Ins2(Akita)Ldlr⁻/⁻ mice were severely hyperglycemic with impaired glucose tolerance. Compared with Ldlr⁻/⁻ mice, 20-week-old Ins2(Akita)Ldlr⁻/⁻ mice fed a 0.02% cholesterol AIN76a diet showed increased plasma triglyceride and cholesterol levels, and increased aortic root cross-sectional atherosclerotic lesion area [224% (P < 0.001) in males and 30% (P < 0.05) in females]. Microarray and quantitative PCR analyses of livers from Ins2(Akita)Ldlr⁻/⁻ mice revealed altered expression of lipid homeostatic genes, including sterol-regulatory element binding protein (Srebp)1, liver X receptor (Lxr)α, Abca1, Cyp7b1, Cyp27a1, and Lpl, along with increased expression of pro-inflammatory cytokine genes, including interleukin (Il)1α, Il1β, Il2, tumor necrosis factor (Tnf)α, and Mcp1. Immunofluorescence staining showed that the expression levels of Mcp1, Tnfα, and Il1β were also increased in the atherosclerotic lesions and artery walls of Ins2(Akita)Ldlr⁻/⁻ mice. Thus, the Ins2(Akita)Ldlr⁻/⁻ mouse appears to be a promising model for mechanistic studies of type 1 diabetes-accelerated atherosclerosis.
Figures



References
-
- Calles-Escandon J., Cipolla M. 2001. Diabetes and endothelial dysfunction: a clinical perspective. Endocr. Rev. 22: 36–52. - PubMed
-
- Retnakaran R., Zinman B. 2008. Type 1 diabetes, hyperglycaemia, and the heart. Lancet. 371: 1790–1799. - PubMed
-
- Roglic G., Unwin N., Bennett P. H., Mathers C., Tuomilehto J., Nag S., Connolly V., King H. 2005. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 28: 2130–2135. - PubMed
-
- Coccheri S. 2007. Approaches to prevention of cardiovascular complications and events in diabetes mellitus. Drugs. 67: 997–1026. - PubMed
-
- Libby P., Nathan D. M., Abraham K., Brunzell J. D., Fradkin J. E., Haffner S. M., Hsueh W., Rewers M., Roberts B. T., Savage P. J., et al. 2005. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on cardiovascular complications of type 1 diabetes mellitus. Circulation. 111: 3489–3493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

