Polyubiquitin binding to ABIN1 is required to prevent autoimmunity
- PMID: 21606507
- PMCID: PMC3173241
- DOI: 10.1084/jem.20102177
Polyubiquitin binding to ABIN1 is required to prevent autoimmunity
Abstract
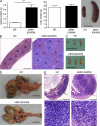
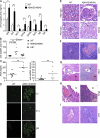
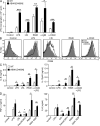
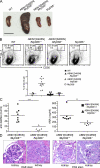
The protein ABIN1 possesses a polyubiquitin-binding domain homologous to that present in nuclear factor κB (NF-κB) essential modulator (NEMO), a component of the inhibitor of NF-κB (IκB) kinase (IKK) complex. To address the physiological significance of polyubiquitin binding, we generated knockin mice expressing the ABIN1[D485N] mutant instead of the wild-type (WT) protein. These mice developed all the hallmarks of autoimmunity, including spontaneous formation of germinal centers, isotype switching, and production of autoreactive antibodies. Autoimmunity was suppressed by crossing to MyD88(-/-) mice, demonstrating that toll-like receptor (TLR)-MyD88 signaling pathways are needed for the phenotype to develop. The B cells and myeloid cells of the ABIN1[D485N] mice showed enhanced activation of the protein kinases TAK, IKK-α/β, c-Jun N-terminal kinases, and p38α mitogen-activated protein kinase and produced more IL-6 and IL-12 than WT. The mutant B cells also proliferated more rapidly in response to TLR ligands. Our results indicate that the interaction of ABIN1 with polyubiquitin is required to limit the activation of TLR-MyD88 pathways and prevent autoimmunity.
Figures



References
-
- Chu Y., Vahl J.C., Kumar D., Heger K., Bertossi A., Wójtowicz E., Soberon V., Schenten D., Mack B., Reutelshöfer M., et al. 2011. B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice. Blood. 117:2227–2236 10.1182/blood-2010-09-306019 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

