A randomized placebo-controlled phase IIb trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation
- PMID: 21606974
- PMCID: PMC3188811
- DOI: 10.1038/ajg.2011.162
A randomized placebo-controlled phase IIb trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation
Erratum in
- Am J Gastroenterol. 2014 May;109(5):782
Abstract
Objectives: A3309 is a minimally absorbed ileal bile acid (BA) transporter (IBAT) inhibitor. We conducted an 8-week, multicenter, randomized, double-blind, placebo-controlled, parallel group, phase IIb study, which evaluated A3309 in patients with chronic idiopathic constipation (CIC).
Methods: Patients with CIC (modified Rome III criteria and <3 complete (CSBM) spontaneous bowel movements (SBMs)/week during the 2-week baseline) were randomized to 5, 10, or 15 mg A3309 or placebo once daily. The primary end point was change in SBM number during week 1 compared with baseline. Other bowel and abdominal symptoms were assessed as secondary end points. Serum 7αC4 and lipids were evaluated as biomarkers of BA synthesis/loss.
Results: In all, 190 patients (mean 48 years, 90% female) were randomized. Mean increase (95% confidence interval) in SBM for week 1 were 1.7 (0.7-2.8) for placebo vs. 2.5 (1.5-3.5), 4.0 (2.9-5.0), and 5.4 (4.4-6.4) for 5 mg, 10 mg (P<0.002), and 15 mg (P<0.001) A3309, respectively. Increased stool frequency was maintained over 8 weeks. Time to first SBM and CSBM were significantly reduced in the 10- and 15-mg A3309 groups compared with placebo. Straining and bloating decreased with A3309 compared with placebo (P<0.05). Increased 7αC4 and reduced low-density lipoprotein cholesterol with A3309 suggested increased BA synthesis and BA loss. The most common adverse events (AEs) were abdominal pain and diarrhea, which occurred most commonly in the 15-mg A3309 group. No drug-related serious AEs were observed.
Conclusions: A3309 increased stool frequency and improved constipation-related symptoms in CIC; effects were maintained over 8 weeks of treatment.
Figures


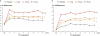

Comment in
-
Altered bile acid pool using IBAT inhibitors for constipation: a potentially increased risk of malignancy.Am J Gastroenterol. 2012 Jan;107(1):140; author reply 140-1. doi: 10.1038/ajg.2011.378. Am J Gastroenterol. 2012. PMID: 22218036 No abstract available.
-
Turning classical bile acid physiology into everyday pharmacology: impact of a bile acid transporter inhibitor on chronic constipation.Gastroenterology. 2012 Jul;143(1):262-4. doi: 10.1053/j.gastro.2012.05.026. Epub 2012 May 23. Gastroenterology. 2012. PMID: 22633768 No abstract available.
-
[To cast out devils by Beelzebub?].Z Gastroenterol. 2012 Jul;50(7):702-3. doi: 10.1055/s-0032-1312906. Epub 2012 Jul 3. Z Gastroenterol. 2012. PMID: 22760684 German. No abstract available.
References
-
- Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil. 2008;20 (Suppl 1:121–129. - PubMed
-
- Higgins PD, Johansen JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–759. - PubMed
-
- Pare P, Ferrazzi S, Thompson WG, et al. An epidemiological survey of constipation in Canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001;96:3130–3137. - PubMed
-
- Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25:599–608. - PubMed
-
- Hofmann AF, Poley JR. Cholestyramine treatment of diarrhea associated with ileal resection. N Engl J Med. 1969;281:397–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

