Gemcitabine enhances Wilms' tumor gene WT1 expression and sensitizes human pancreatic cancer cells with WT1-specific T-cell-mediated antitumor immune response
- PMID: 21607557
- PMCID: PMC11029139
- DOI: 10.1007/s00262-011-1033-3
Gemcitabine enhances Wilms' tumor gene WT1 expression and sensitizes human pancreatic cancer cells with WT1-specific T-cell-mediated antitumor immune response
Abstract
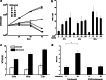
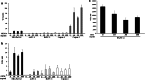
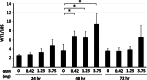
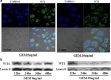
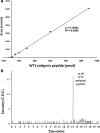
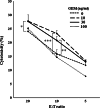
Wilms' tumor gene (WT1), which is expressed in human pancreatic cancer (PC), is a unique tumor antigen recognized by T-cell-mediated antitumor immune response. Gemcitabine (GEM), a standard therapeutic drug for PC, was examined for the regulation of WT1 expression and the sensitizing effect on PC cells with WT1-specific antitumor immune response. Expression of WT1 was examined by quantitative PCR, immunoblot analysis, and confocal microscopy. Antigenic peptide of WT1 presented on HLA class I molecules was detected by mass spectrometry. WT1-specific T-cell receptor gene-transduced human T cells were used as effecter T cells for the analysis of cytotoxic activity. GEM treatment of human MIAPaCa2 PC cells enhanced WT1 mRNA levels, and this increase is associated with nuclear factor kappa B activation. Tumor tissue from GEM-treated MIAPaCa2-bearing SCID mice also showed an increase in WT1 mRNA. Some human PC cell lines other than MIAPaCa2 showed up-regulation of WT1 mRNA levels following GEM treatment. GEM treatment shifted WT1 protein from the nucleus to the cytoplasm, which may promote proteasomal processing of WT1 protein and generation of antigenic peptide. In fact, presentation of HLA-A*2402-restricted antigenic peptide of WT1 (CMTWNQMNL) increased in GEM-treated MIAPaCa2 cells relative to untreated cells. WT1-specific cytotoxic T cells killed MIAPaCa2 cells treated with an optimal dose of GEM more efficiently than untreated MIAPaCa2 cells. GEM enhanced WT1 expression in human PC cells and sensitized PC cells with WT1-specific T-cell-mediated antitumor immune response.
Conflict of interest statement
There are no financial disclosures of any of the authors to declare.
Figures

References
-
- Heinemann V. Gemcitabine in the treatment of advanced pancreatic cancer: a comparative analysis of randomized trials. Semin Oncol. 2002;29(6 Suppl20):9–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

