TDP-43 variants of frontotemporal lobar degeneration
- PMID: 21607722
- PMCID: PMC3202017
- DOI: 10.1007/s12031-011-9545-z
TDP-43 variants of frontotemporal lobar degeneration
Abstract
It has been only 5 years since the identification of TDP-43 as the major protein component of the ubiquitinated inclusions in FTLD-U. At that time, there were approximately a dozen papers about TDP-43; today, a "TDP-43" search reveals almost 600 papers. It is now clear that the majority of FTLD cases containing tau- and alpha-synuclein-negative, ubiquitin-positive inclusions (FTLD-U) are FTLD-TDP. The spectrum of TDP-43 proteinopathies includes FTLD-TDP with or without ALS, with or without mutations in GRN, VCP, or TARDBP, with or without chromosome 9p linkage, and sporadic and non-SOD1 familial ALS with or without FTLD-TDP. There are four sub-types of FTLD-TDP, and these correlate with specific clinical and genetic profiles. Sub-types are determined by the presence, predominance, and distribution of the various TDP-43 immunopositive insoluble aggregates-neuronal cytoplasmic inclusions, neuronal intranuclear inclusions, and dystrophic neurites. In this paper, FTLD-TDP pathologic sub-types will be described, and examples of each sub-type will be shown, and implications for future research will be discussed.
Figures








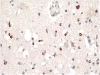





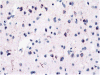
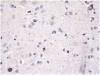


References
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. - PubMed
-
- Armstrong RA, Ellis W, Hamilton RL, Mackenzie IRA, Hedreen J, Gearing M, Montine T, Vonsattel J-P, Head E, Lieberman AP, Cairns NJ. Neuropathological heterogeneity in frontotemporal lobar degeneration with TDP-43 proteinopathy: a quantitative study of 94 cases using principal components analysis. J Neural Transm. 2010;117:227–239. - PMC - PubMed
-
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick D, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. - PubMed
-
- Borroni B, Bonvicini C, Alberici A, Buratti E, Agosti C, Archetti S, Papetti A, Stuani C, Di Luca M, Gennarelli M, Padovani A. Mutation within TARDBP leads to frontotemporal dementia without motor neuron disease. Human Mutat. 2009;30:E974–E983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

