Amino acid analog toxicity in primary rat neuronal and astrocyte cultures: implications for protein misfolding and TDP-43 regulation
- PMID: 21608013
- PMCID: PMC3175609
- DOI: 10.1002/jnr.22677
Amino acid analog toxicity in primary rat neuronal and astrocyte cultures: implications for protein misfolding and TDP-43 regulation
Abstract
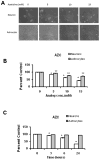
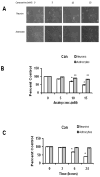
Amino acid analogs promote translational errors that result in aberrant protein synthesis and have been used to understand the effects of protein misfolding in a variety of physiological and pathological settings. TDP-43 is a protein that is linked to protein aggregation and toxicity in a variety of neurodegenerative diseases. This study exposed primary rat neurons and astrocyte cultures to established amino acid analogs (canavanine and azetidine-2-carboxylic acid) and showed that both cell types undergo a dose-dependent increase in toxicity, with neurons exhibiting a greater degree of toxicity compared with astrocytes. Neurons and astrocytes exhibited similar increases in ubiquitinated and oxidized protein following analog treatment. Analog treatment increased heat shock protein (Hsp) levels in both neurons and astrocytes. In neurons, and to a lesser extent astrocytes, the levels of TDP-43 increased in response to analog treatment. Taken together, these data indicate that neurons exhibit preferential toxicity and alterations in TDP-43 in response to increased protein misfolding compared with astrocytes.
Copyright © 2011 Wiley-Liss, Inc.
Conflict of interest statement
The authors declare that they do not have any conflict of interests.
Figures



Similar articles
-
Misfolded proteins are competent to mediate a subset of the responses to heat shock in Saccharomyces cerevisiae.J Biol Chem. 2002 Nov 22;277(47):44817-25. doi: 10.1074/jbc.M204686200. Epub 2002 Sep 17. J Biol Chem. 2002. PMID: 12239211
-
Selective vulnerability of neurons to acute toxicity after proteasome inhibitor treatment: implications for oxidative stress and insolubility of newly synthesized proteins.Free Radic Biol Med. 2010 Nov 1;49(8):1290-7. doi: 10.1016/j.freeradbiomed.2010.07.014. Epub 2010 Aug 1. Free Radic Biol Med. 2010. PMID: 20678570 Free PMC article.
-
Hsp90 and its co-chaperone Sti1 control TDP-43 misfolding and toxicity.FASEB J. 2021 May;35(5):e21594. doi: 10.1096/fj.202002645R. FASEB J. 2021. PMID: 33908654
-
N-Acetyl-l-Cysteine Protects Astrocytes against Proteotoxicity without Recourse to Glutathione.Mol Pharmacol. 2017 Nov;92(5):564-575. doi: 10.1124/mol.117.109926. Epub 2017 Aug 22. Mol Pharmacol. 2017. PMID: 28830914 Free PMC article.
-
Monocarboxylate transporter-dependent mechanism confers resistance to oxygen- and glucose-deprivation injury in astrocyte-neuron co-cultures.Neurosci Lett. 2015 May 6;594:99-104. doi: 10.1016/j.neulet.2015.03.062. Epub 2015 Mar 28. Neurosci Lett. 2015. PMID: 25827488
Cited by
-
The Ubiquitin Ligase CHIP Integrates Proteostasis and Aging by Regulation of Insulin Receptor Turnover.Cell. 2017 Apr 20;169(3):470-482.e13. doi: 10.1016/j.cell.2017.04.003. Cell. 2017. PMID: 28431247 Free PMC article.
-
Intracellular fibril formation, calcification, and enrichment of chaperones, cytoskeletal, and intermediate filament proteins in the adult hippocampus CA1 following neonatal exposure to the nonprotein amino acid BMAA.Arch Toxicol. 2015 Mar;89(3):423-36. doi: 10.1007/s00204-014-1262-2. Epub 2014 May 6. Arch Toxicol. 2015. PMID: 24798087 Free PMC article.
-
Pharmacological inhibition of USP14 delays proteostasis-associated aging in a proteasome-dependent but foxo-independent manner.Autophagy. 2024 Dec;20(12):2752-2768. doi: 10.1080/15548627.2024.2389607. Epub 2024 Aug 15. Autophagy. 2024. PMID: 39113571 Free PMC article.
-
NFκB is a central regulator of protein quality control in response to protein aggregation stresses via autophagy modulation.Mol Biol Cell. 2016 Jun 1;27(11):1712-27. doi: 10.1091/mbc.E15-12-0835. Epub 2016 Apr 13. Mol Biol Cell. 2016. PMID: 27075172 Free PMC article.
References
-
- Agorogiannis EI, Agorogiannis GI, Papadimitriou A, Hadjigeorgiou GM. Protein misfolding in neurodegenerative diseases. N europathol Appl Neurobiol. 2004;30:215–224. - PubMed
-
- Ananthan J, Goldberg AL, Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986;232:522–524. - PubMed
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. - PubMed
-
- Barrett MJ, Alones V, Wang KX, Phan L, Swerdlow RH. Mitochondria-derived oxidative stress induces a heat shock protein response. J Neurosci Res. 2004;78:420–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

