Development of histocompatible primate-induced pluripotent stem cells for neural transplantation
- PMID: 21608081
- PMCID: PMC3340906
- DOI: 10.1002/stem.662
Development of histocompatible primate-induced pluripotent stem cells for neural transplantation
Abstract
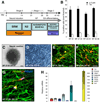
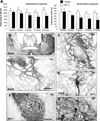
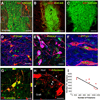
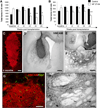
Immune rejection and risk of tumor formation are perhaps the greatest hurdles in the field of stem cell transplantation. Here, we report the generation of several lines of induced pluripotent stem cells (iPSCs) from cynomolgus macaque (CM) skin fibroblasts carrying specific major histocompatibility complex (MHC) haplotypes. To develop a collection of MHC-matched iPSCs, we genotyped the MHC locus of 25 CMs by microsatellite polymerase chain reaction analysis. Using retroviral infection of dermal skin fibroblasts, we generated several CM-iPSC lines carrying different haplotypes. We characterized the immunological properties of CM-iPSCs and demonstrated that CM-iPSCs can be induced to differentiate in vitro along specific neuronal populations, such as midbrain dopaminergic (DA) neurons. Midbrain-like DA neurons generated from CM-iPSCs integrated into the striatum of a rodent model of Parkinson's disease and promoted behavioral recovery. Importantly, neither tumor formation nor inflammatory reactions were observed in the transplanted animals up to 6 months after transplantation. We believe that the generation and characterization of such histocompatible iPSCs will allow the preclinical validation of safety and efficacy of iPSCs for neurodegenerative diseases and several other human conditions in the field of regenerative medicine.
Copyright © 2011 AlphaMed Press.
Conflict of interest statement
Figures


References
-
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, N.Y. 2007;318:1917–1920. - PubMed
-
- Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. - PubMed
-
- Nakatsuji N, Nakajima F, Tokunaga K. HLA-haplotype banking and iPS cells. Nature biotechnology. 2008;26:739–740. - PubMed
-
- Bontrop RE. Non-human primates: essential partners in biomedical research. Immunological reviews. 2001;183:5–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

