Photoinducible bioorthogonal chemistry: a spatiotemporally controllable tool to visualize and perturb proteins in live cells
- PMID: 21609129
- PMCID: PMC3175026
- DOI: 10.1021/ar200021p
Photoinducible bioorthogonal chemistry: a spatiotemporally controllable tool to visualize and perturb proteins in live cells
Abstract
Visualization in biology has been greatly facilitated by the use of fluorescent proteins as in-cell probes. The genes coding for these wavelength-tunable proteins can be readily fused with the DNA coding for a protein of interest, which enables direct monitoring of natural proteins in real time inside living cells. Despite their success, however, fluorescent proteins have limitations that have only begun to be addressed in the past decade through the development of bioorthogonal chemistry. In this approach, a very small bioorthogonal tag is embedded within the basic building blocks of the cell, and then a variety of external molecules can be selectively conjugated to these pretagged biomolecules. The result is a veritable palette of biophysical probes for the researcher to choose from. In this Account, we review our progress in developing a photoinducible, bioorthogonal tetrazole-alkene cycloaddition reaction ("photoclick chemistry") and applying it to probe protein dynamics and function in live cells. The work described here summarizes the synthesis, structure, and reactivity studies of tetrazoles, including their optimization for applications in biology. Building on key insights from earlier reports, our initial studies of the reaction have revealed full water compatibility, high photoactivation quantum yield, tunable photoactivation wavelength, and broad substrate scope; an added benefit is the formation of fluorescent cycloadducts. Subsequent studies have shown fast reaction kinetics (up to 11.0 M(-1) s(-1)), with the rate depending on the HOMO energy of the nitrile imine dipole as well as the LUMO energy of the alkene dipolarophile. Moreover, through the use of photocrystallography, we have observed that the photogenerated nitrile imine adopts a bent geometry in the solid state. This observation has led to the synthesis of reactive, macrocyclic tetrazoles that contain a short "bridge" between two flanking phenyl rings. This photoclick chemistry has been used to label proteins rapidly (within ∼1 min) both in vitro and in E. coli . To create an effective interface with biology, we have identified both a metabolically incorporable alkene amino acid, homoallylglycine, and a genetically encodable tetrazole amino acid, p-(2-tetrazole)phenylalanine. We demonstrate the utility of these two moieties, respectively, in spatiotemporally controlled imaging of newly synthesized proteins and in site-specific labeling of proteins. Additionally, we demonstrate the use of the photoclick chemistry to perturb the localization of a fluorescent protein in mammalian cells.
© 2011 American Chemical Society
Figures









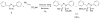
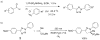


References
-
- Tsien RY. Constructing and exploiting the fluorescent protein paintbox (Nobel Lecture) Angew Chem Int Ed. 2009;48:5612–5626. - PubMed
-
- Moritz OL, Tam BM, Papermaster DS, Nakayama T. A Functional Rhodopsin-Green Fluorescent Protein Fusion Protein Localizes Correctly in Transgenic Xenopus laevis Retinal Rods and Is Expressed in a Time-dependent Pattern. J Biol Chem. 2001;276:28242–28251. - PubMed
-
- Marsh DR, Holmes KD, Dekaban GA, Weaver LC. Distribution of an NMDA receptor:GFP fusion protein in sensory neurons is altered by a C-terminal construct. J Neurochem. 2001;77:23–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

