Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results
- PMID: 21609134
- PMCID: PMC3205788
- DOI: 10.1089/hum.2011.053
Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results
Abstract
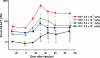
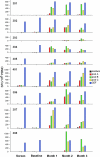
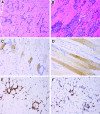
Recombinant adeno-associated virus (rAAV) vectors offer promise for the gene therapy of α(1)-antitrypsin (AAT) deficiency. In our prior trial, an rAAV vector expressing human AAT (rAAV1-CB-hAAT) provided sustained, vector-derived AAT expression for >1 year. In the current phase 2 clinical trial, this same vector, produced by a herpes simplex virus complementation method, was administered to nine AAT-deficient individuals by intramuscular injection at doses of 6.0×10(11), 1.9×10(12), and 6.0×10(12) vector genomes/kg (n=3 subjects/dose). Vector-derived expression of normal (M-type) AAT in serum was dose dependent, peaked on day 30, and persisted for at least 90 days. Vector administration was well tolerated, with only mild injection site reactions and no serious adverse events. Serum creatine kinase was transiently elevated on day 30 in five of six subjects in the two higher dose groups and normalized by day 45. As expected, all subjects developed anti-AAV antibodies and interferon-γ enzyme-linked immunospot responses to AAV peptides, and no subjects developed antibodies to AAT. One subject in the mid-dose group developed T cell responses to a single AAT peptide unassociated with any clinical effects. Muscle biopsies obtained on day 90 showed strong immunostaining for AAT and moderate to marked inflammatory cell infiltrates composed primarily of CD3-reactive T lymphocytes that were primarily of the CD8(+) subtype. These results support the feasibility and safety of AAV gene therapy for AAT deficiency, and indicate that serum levels of vector-derived normal human AAT >20 μg/ml can be achieved. However, further improvements in the design or delivery of rAAV-AAT vectors will be required to achieve therapeutic target serum AAT concentrations.
Trial registration: ClinicalTrials.gov NCT01054339.
Figures

Comment in
-
Adeno-associated viral vectors for hereditary emphysema; progressing along the clinical development highway.Hum Gene Ther. 2011 Oct;22(10):1175-7. doi: 10.1089/hum.2011.2524. Hum Gene Ther. 2011. PMID: 22044094 No abstract available.
References
-
- Brantly M.L. Wittes J.T. Vogelmeier C.F., et al. Use of a highly purified α1-antitrypsin standard to establish ranges for the common normal and deficient α1-antitrypsin phenotypes. Chest. 1991;100:703–708. - PubMed
-
- Brantly M.L. Spencer L.T. Humphries M., et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 α1-antitrypsin (AAT) vector in AAT-deficient adults. Hum. Gene Ther. 2006;17:1177–1186. - PubMed
-
- Chao H. Liu Y. Rabinowitz J., et al. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2000;2:619–623. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

