Carboxylesterase inhibitors
- PMID: 21609191
- PMCID: PMC3139797
- DOI: 10.1517/13543776.2011.586339
Carboxylesterase inhibitors
Abstract
Introduction: Carboxylesterases play major roles in the hydrolysis of numerous therapeutically active compounds. This is, in part, due to the prevalence of the ester moiety in these small molecules. However, the impact these enzymes may play on drug stability and pharmacokinetics is rarely considered prior to molecule development. Therefore, the application of selective inhibitors of this class of proteins may have utility in modulating the metabolism, distribution and toxicity of agents that are subjected to enzyme hydrolysis.
Areas covered: This review details the development of all such compounds dating back to 1986, but principally focuses on the very recent identification of selective human carboxylesterases inhibitors.
Expert opinion: The implementation of carboxylesterase inhibitors may significantly revolutionize drug discovery. Such molecules may allow for improved efficacy of compounds inactivated by this class of enzymes and/or reduce the toxicity of agents that are activated by these proteins. Furthermore, since lack of carboxylesterase activity appears to have no obvious biological consequence, these compounds could be applied in combination with virtually any esterified drug. Therefore, inhibitors of these proteins may have utility in altering drug hydrolysis and distribution in vivo. The characteristics, chemical and biological properties and potential uses of such agents are discussed here.
Conflict of interest statement
Figures
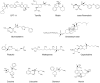
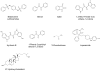
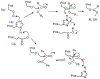

References
-
- Potter PM, Wadkins RM. Carboxylesterases – detoxifying enzymes and targets for drug therapy. Curr Med Chem. 2006;13:1045–1054. - PubMed
-
- Redinbo MR, Potter PM. Mammalian Carboxylesterases: From drug targets to protein therapeutics. Drug Discov Today. 2005;10:313–325. - PubMed
-
- Bencharit S, Edwards CC, Morton CL, et al. Multisite promiscuity in the processing of endogenous substrates by human carboxylesterase 1. J Mol Biol. 2006;363:201–214. This paper details the plasticity of the active site of a human carboxylesterase that accounts for the very diverse substrate specificity of this protein. - PMC - PubMed
-
- Humerickhouse R, Lohrbach K, Li L, et al. Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Res. 2000;60:1189–1192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
