Exploring the metabolic network of the epidemic pathogen Burkholderia cenocepacia J2315 via genome-scale reconstruction
- PMID: 21609491
- PMCID: PMC3123600
- DOI: 10.1186/1752-0509-5-83
Exploring the metabolic network of the epidemic pathogen Burkholderia cenocepacia J2315 via genome-scale reconstruction
Abstract
Background: Burkholderia cenocepacia is a threatening nosocomial epidemic pathogen in patients with cystic fibrosis (CF) or a compromised immune system. Its high level of antibiotic resistance is an increasing concern in treatments against its infection. Strain B. cenocepacia J2315 is the most infectious isolate from CF patients. There is a strong demand to reconstruct a genome-scale metabolic network of B. cenocepacia J2315 to systematically analyze its metabolic capabilities and its virulence traits, and to search for potential clinical therapy targets.
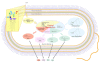
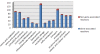
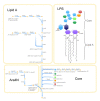
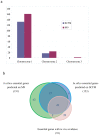
Results: We reconstructed the genome-scale metabolic network of B. cenocepacia J2315. An iterative reconstruction process led to the establishment of a robust model, iKF1028, which accounts for 1,028 genes, 859 internal reactions, and 834 metabolites. The model iKF1028 captures important metabolic capabilities of B. cenocepacia J2315 with a particular focus on the biosyntheses of key metabolic virulence factors to assist in understanding the mechanism of disease infection and identifying potential drug targets. The model was tested through BIOLOG assays. Based on the model, the genome annotation of B. cenocepacia J2315 was refined and 24 genes were properly re-annotated. Gene and enzyme essentiality were analyzed to provide further insights into the genome function and architecture. A total of 45 essential enzymes were identified as potential therapeutic targets.
Conclusions: As the first genome-scale metabolic network of B. cenocepacia J2315, iKF1028 allows a systematic study of the metabolic properties of B. cenocepacia and its key metabolic virulence factors affecting the CF community. The model can be used as a discovery tool to design novel drugs against diseases caused by this notorious pathogen.
Figures
References
-
- Vanlaere E, Lipuma JJ, Baldwin A, Henry D, De Brandt E, Mahenthiralingam E, Speert D, Dowson C, Vandamme P. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int J Syst Evol Microbiol. 2008;58:1580–1590. doi: 10.1099/ijs.0.65634-0. - DOI - PubMed
-
- Vanlaere E, Baldwin A, Gevers D, Henry D, De Brandt E, LiPuma JJ, Mahenthiralingam E, Speert DP, Dowson C, Vandamme P. Taxon K, a complex within the Burkholderia cepacia complex, comprises at least two novel species, Burkholderia contaminans sp. nov. and Burkholderia lata sp. nov. Int J Syst Evol Microbiol. 2009;59:102–111. doi: 10.1099/ijs.0.001123-0. - DOI - PubMed
-
- Mahenthiralingam E, Baldwin A, Vandamme P. Burkholderia cepacia complex infection in patients with cystic fibrosis. J Med Microbiol. 2002;51:533–538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

