Neural stem cells: historical perspective and future prospects
- PMID: 21609820
- PMCID: PMC3225274
- DOI: 10.1016/j.neuron.2011.05.005
Neural stem cells: historical perspective and future prospects
Abstract
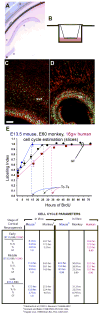
How a single fertilized cell generates diverse neuronal populations has been a fundamental biological problem since the 19(th) century. Classical histological methods revealed that postmitotic neurons are produced in a precise temporal and spatial order from germinal cells lining the cerebral ventricles. In the 20(th) century, DNA labeling and histo- and immunohistochemistry helped to distinguish the subtypes of dividing cells and delineate their locations in the ventricular and subventricular zones. Recently, genetic and cell biological methods have provided insights into sequential gene expression and molecular and cellular interactions that generate heterogeneous populations of NSCs leading to specific neuronal classes. This precisely regulated developmental process does not tolerate significant in vivo deviation, making replacement of adult neurons by NSCs during pathology a colossal challenge. In contrast, utilizing the trophic factors emanating from the NSC or their derivatives to slow down deterioration or prevent death of degenerating neurons may be a more feasible strategy.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Altman J. ARE NEW NEURONS FORMED IN BRAINS OF ADULT MAMMALS. Science. 1962;135:1127. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
-
- Alvarez-Dolado M, Pardal R, Garcia-Vardugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

