The MADS box transcription factor MEF2C regulates melanocyte development and is a direct transcriptional target and partner of SOX10
- PMID: 21610032
- PMCID: PMC3100711
- DOI: 10.1242/dev.056804
The MADS box transcription factor MEF2C regulates melanocyte development and is a direct transcriptional target and partner of SOX10
Abstract
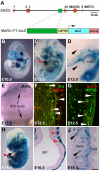
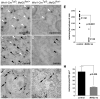
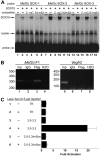
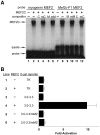
Waardenburg syndromes are characterized by pigmentation and autosensory hearing defects, and mutations in genes encoding transcription factors that control neural crest specification and differentiation are often associated with Waardenburg and related disorders. For example, mutations in SOX10 result in a severe form of Waardenburg syndrome, Type IV, also known as Waardenburg-Hirschsprung disease, characterized by pigmentation and other neural crest defects, including defective innervation of the gut. SOX10 controls neural crest development through interactions with other transcription factors. The MADS box transcription factor MEF2C is an important regulator of brain, skeleton, lymphocyte and cardiovascular development and is required in the neural crest for craniofacial development. Here, we establish a novel role for MEF2C in melanocyte development. Inactivation of Mef2c in the neural crest of mice results in reduced expression of melanocyte genes during development and a significant loss of pigmentation at birth due to defective differentiation and reduced abundance of melanocytes. We identify a transcriptional enhancer of Mef2c that directs expression to the neural crest and its derivatives, including melanocytes, in transgenic mouse embryos. This novel Mef2c neural crest enhancer contains three functional SOX binding sites and a single essential MEF2 site. We demonstrate that Mef2c is a direct transcriptional target of SOX10 and MEF2 via this evolutionarily conserved enhancer. Furthermore, we show that SOX10 and MEF2C physically interact and function cooperatively to activate the Mef2c gene in a feed-forward transcriptional circuit, suggesting that MEF2C might serve as a potentiator of the transcriptional pathways affected in Waardenburg syndromes.
Figures



References
-
- Baker C. V., Bronner-Fraser M. (1997). The origins of the neural crest. Part I: embryonic induction. Mech. Dev. 69, 3-11. - PubMed
-
- Baxter L. L., Hou L., Loftus S. K., Pavan W. J. (2004). Spotlight on spotted mice: a review of white spotting mouse mutants and associated human pigmentation disorders. Pigment Cell Res. 17, 215-224. - PubMed
-
- Bi W., Drake C. J., Schwarz J. J. (1999). The transcription factor MEF2C-null mouse exhibits complex vascular malformations and reduced cardiac expression of angiopoietin 1 and VEGF. Dev. Biol. 211, 255-267. - PubMed
-
- Black B. L., Cripps R. M. (2010). Myocyte enhancer factor 2 transcription factors in heart development and disease. In Heart Development and Regeneration, Vol. 2 (ed. Rosenthal N., Harvey R. P.), pp. 673-699. Oxford: Academic Press.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

