Steroidogenic factor-1 (SF-1)-driven differentiation of murine embryonic stem (ES) cells into a gonadal lineage
- PMID: 21610156
- PMCID: PMC3192422
- DOI: 10.1210/en.2011-0219
Steroidogenic factor-1 (SF-1)-driven differentiation of murine embryonic stem (ES) cells into a gonadal lineage
Abstract
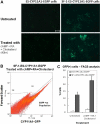
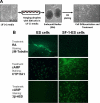
Steroidogenic factor 1 (SF-1) is essential for the development and function of steroidogenic tissues. Stable incorporation of SF-1 into embryonic stem cells (SF-1-ES cells) has been shown to prime the cells for steroidogenesis. When provided with exogenous cholesterol substrate, and after treatment with retinoic acid and cAMP, SF-1-ES cells produce progesterone but do not produce other steroids such as cortisol, estradiol, or testosterone. In this study, we explored culture conditions that optimize SF-1-mediated differentiation of ES cells into defined steroidogenic lineages. When embryoid body formation was used to facilitate cell lineage differentiation, SF-1-ES cells were found to be restricted in their differentiation, with fewer cells entering neuronal pathways and a larger fraction entering the steroidogenic lineage. Among the differentiation protocols tested, leukemia inhibitory factor (LIF) removal, followed by prolonged cAMP treatment was most efficacious for inducing steroidogenesis in SF-1-ES cells. In this protocol, a subset of SF-1-ES cells survives after LIF withdrawal, undergoes morphologic differentiation, and recovers proliferative capacity. These cells are characterized by induction of steroidogenic enzyme genes, use of de novo cholesterol, and production of multiple steroids including estradiol and testosterone. Microarray studies identified additional pathways associated with SF-1 mediated differentiation. Using biotinylated SF-1 in chromatin immunoprecipitation assays, SF-1 was shown to bind directly to multiple target genes, with induction of binding to some targets after steroidogenic treatment. These studies indicate that SF-1 expression, followed by LIF removal and treatment with cAMP drives ES cells into a steroidogenic pathway characteristic of gonadal steroid-producing cells.
Figures






References
-
- Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL. 1993. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol 7:852–860 - PubMed
-
- Morohashi K, Honda S, Inomata Y, Handa H, Omura T. 1992. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem 267:17913–17919 - PubMed
-
- Morohashi K, Zanger UM, Honda S, Hara M, Waterman MR, Omura T. 1993. Activation of CYP11A and CYP11B gene promoters by the steroidogenic cell-specific transcription factor, Ad4BP. Mol Endocrinol 7:1196–1204 - PubMed
-
- Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. 1995. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol 9:478–486 - PubMed
-
- Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J. 1995. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA 92:10939–10943 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

