The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis
- PMID: 21610183
- PMCID: PMC3123942
- DOI: 10.1105/tpc.110.081943
The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis
Abstract
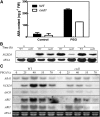
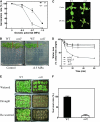
Osmotic stress activates the biosynthesis of abscisic acid (ABA). One major step in ABA biosynthesis is the carotenoid cleavage catalyzed by a 9-cis epoxycarotenoid dioxygenase (NCED). To understand the mechanism for osmotic stress activation of ABA biosynthesis, we screened for Arabidopsis thaliana mutants that failed to induce the NCED3 gene expression in response to osmotic stress treatments. The ced1 (for 9-cis epoxycarotenoid dioxygenase defective 1) mutant isolated in this study showed markedly reduced expression of NCED3 in response to osmotic stress (polyethylene glycol) treatments compared with the wild type. Other ABA biosynthesis genes are also greatly reduced in ced1 under osmotic stress. ced1 mutant plants are very sensitive to even mild osmotic stress. Map-based cloning revealed unexpectedly that CED1 encodes a putative α/β hydrolase domain-containing protein and is allelic to the BODYGUARD gene that was recently shown to be essential for cuticle biogenesis. Further studies discovered that other cutin biosynthesis mutants are also impaired in osmotic stress induction of ABA biosynthesis genes and are sensitive to osmotic stress. Our work demonstrates that the cuticle functions not merely as a physical barrier to minimize water loss but also mediates osmotic stress signaling and tolerance by regulating ABA biosynthesis and signaling.
Figures




References
-
- Bermejo C., Rodriguez E., Garcia R., Rodriguez-Pena J.M., Rodriguez de la Concepcion M.L., Rivas C., Arias P., Nombela C., Posas F., Arroyo J. (2008). The sequential activation of the yeast HOG and SLT2 pathways is required for cell survival to cell wall stress. Mol. Biol. Cell 19: 1113–1124 - PMC - PubMed
-
- Bianco-Trinchant J., Le Page-Degivry M.T. (1998). ABA synthesis in protoplasts of different origin in response to osmotic stress. Plant Growth Regul. 25: 135–141
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

