Envelope-chimeric entry-targeted measles virus escapes neutralization and achieves oncolysis
- PMID: 21610701
- PMCID: PMC3188758
- DOI: 10.1038/mt.2011.92
Envelope-chimeric entry-targeted measles virus escapes neutralization and achieves oncolysis
Abstract
Measles virus (MV) is a promising vector for cancer therapy and multivalent vaccination, but high prevalence of pre-existing neutralizing antibodies may reduce therapeutic efficacy, particularly following systemic administration. MV has only one serotype, but here we show that its envelope glycoproteins can be exchanged with those of the closely related canine distemper virus (CDV), generating a chimeric virus capable of escaping neutralization. To target its entry, we displayed on the CDV attachment protein a single-chain antibody specific for a designated receptor. To enhance oncolytic efficacy we armed the virus with a prodrug convertase gene capable of locally activating chemotherapeutic prodrugs. The new virus achieved high titers, was genetically stable, and was resistant to neutralization by sera from both MV-immunized mice and MV-immune humans. The new virus targeted syngeneic murine tumor cells expressing the designated receptor implanted in immunocompetent mice, and synergized with a chemotherapeutic prodrug in a model of oncolysis. Importantly, the chimeric MV remained oncolytic when administered systemically even in the presence of anti-MV antibodies capable of abrogating the therapeutic efficacy of the parental, nonshielded MV. This work shows that targeting, arming, and shielding can be combined to generate a tumor-specific, neutralization-resistant virus that can synergize with chemotherapeutics.
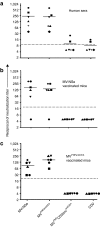
Figures

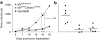
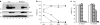

References
-
- Liu TC, Galanis E., and, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. - PubMed
-
- Kumar S, Gao L, Yeagy B., and, Reid T. Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther. 2008;10:371–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

