Induction chemotherapy with paclitaxel, ifosfamide, and cisplatin followed by concurrent chemoradiotherapy for unresectable locally advanced head and neck cancer
- PMID: 21611042
- PMCID: PMC3097775
- DOI: 10.2349/biij.6.3.e23
Induction chemotherapy with paclitaxel, ifosfamide, and cisplatin followed by concurrent chemoradiotherapy for unresectable locally advanced head and neck cancer
Abstract
Objective: Induction chemotherapy (IC) and concurrent chemoradiotherapy (CCRT) for locally advanced head and neck cancer has been studied in many clinical trials. This study was conducted to determine the response rate of IC with paclitaxel, ifosfamide, and cisplatin followed by CCRT with cisplatin for this group of patients, and the effect of the entire treatment on survival and time to disease progression.
Methods: Thirty patients with advanced and unresectable head and neck cancer were treated with 2 cycles of induction paclitaxel/ ifosfamide/ cisplatin. If the primary tumor had a complete or partial response, patients were treated with 2 more cycles of IC followed by radiotherapy 70 Gy plus 3 cycles of cisplatin. For those with less than partial response or disease progression were treated according to the discretion of the physicians.
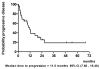
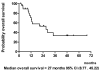
Results: Ninety percent of patients had stage IV disease and 40% of them had primary tumor at maxillary sinus and nasal cavity. One patient (3%) achieved complete response (CR) and 18 patients had partial responses (PR) to IC. CCRT enhanced the response rate, resulting in a total of 3 CR (10%) and 16 PR (53%) to treatment. The median time to progression was 11.5 months. The median overall survival was 27 months. The most severe hematologic toxicity occurred during IC was grade3-4 neutropenia (40%). Grade 3-4 mucositis occurred in 68% of patients during CCRT.
Conclusion: This novel combined-modality treatment program, is toxic but feasible, and can be administered for selected patients with advanced and unresectable head and neck cancer. © 2010 Biomedical Imaging and Intervention Journal. All rights reserved.
Keywords: cisplatin; concurrent chemoradiotherapy; head and neck cancer; ifosfamide; induction chemotherapy; paclitaxel.
Figures
References
-
- Wolf GT. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324(24):1685–90. - PubMed
-
- Paccagnella A, Orlando A, Marchiori C, et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. 1994;86(4):265–72. - PubMed
-
- Lefebvre JL, Chevalier D, Luboinski B, et al. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88(13):890–9. - PubMed
-
- Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355(9208):949–55. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials