Wnt3a induces myofibroblast differentiation by upregulating TGF-β signaling through SMAD2 in a β-catenin-dependent manner
- PMID: 21611174
- PMCID: PMC3097192
- DOI: 10.1371/journal.pone.0019809
Wnt3a induces myofibroblast differentiation by upregulating TGF-β signaling through SMAD2 in a β-catenin-dependent manner
Abstract
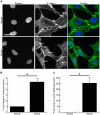
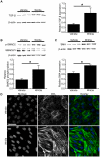
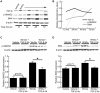
Growing evidence suggests the Wnt family of secreted glycoproteins and their associated signaling pathways, linked to development, are recapitulated during wound repair and regeneration events. However, the role of the Wnt pathway in such settings remains unclear. In the current study, we treated mouse fibroblasts with 250 ng/mL of recombinant Wnt3a for 72 hours and examined its affect on cell morphology and function. Wnt3a induced a spindle-like morphology in fibroblasts characterized by the increased formation of stress fibres. Wnt3a decreased the proliferation of fibroblasts, but significantly increased cell migration as well as fibroblast-mediated contraction of a collagen lattice. Wnt3a significantly increased the expression of TGF-β and its associated signaling through SMAD2. Consistent with this, we observed significantly increased smooth muscle α-actin expression and incorporation of this contractile protein into stress fibres following Wnt3a treatment. Knockdown of β-catenin using siRNA reversed the Wnt3a-induced smooth muscle α-actin expression, suggesting these changes were dependent on canonical Wnt signaling through β-catenin. Neutralization of TGF-β with a blocking antibody significantly inhibited the Wnt3a-induced smooth muscle α-actin expression, indicating these changes were dependent on the increased TGF-β signaling. Collectively, this data strongly suggests Wnt3a promotes the formation of a myofibroblast-like phenotype in cultured fibroblasts, in part, by upregulating TGF-β signaling through SMAD2 in a β-catenin-dependent mechanism. As myofibroblasts are critical regulators of wound healing responses, these findings may have important implications for our understanding of normal and aberrant injury and repair events.
Conflict of interest statement
Figures



References
-
- Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. - PubMed
-
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. - PubMed
-
- Zhao J, Kim KA, Abo A. Tipping the balance: modulating the Wnt pathway for tissue repair. Trends Biotechnol. 2009;27:131–136. - PubMed
-
- Zhang DL, Gu LJ, Liu L, Wang CY, Sun BS, et al. Effect of Wnt signaling pathway on wound healing. Biochem Biophys Res Commun. 2009;378:149–151. - PubMed
-
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

