Normal and abnormal epithelial differentiation in the female reproductive tract
- PMID: 21612855
- PMCID: PMC3178098
- DOI: 10.1016/j.diff.2011.04.008
Normal and abnormal epithelial differentiation in the female reproductive tract
Abstract
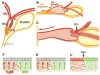
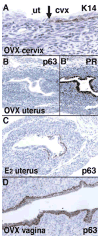
In mammals, the female reproductive tract (FRT) develops from a pair of paramesonephric or Müllerian ducts (MDs), which arise from coelomic epithelial cells of mesodermal origin. During development, the MDs undergo a dynamic morphogenetic transformation from simple tubes consisting of homogeneous epithelium and surrounding mesenchyme into several distinct organs namely the oviduct, uterus, cervix and vagina. Following the formation of anatomically distinctive organs, the uniform MD epithelium (MDE) differentiates into diverse epithelial cell types with unique morphology and functions in each organ. Classic tissue recombination studies, in which the epithelium and mesenchyme isolated from the newborn mouse FRT were recombined, have established that the organ specific epithelial cell fate of MDE is dictated by the underlying mesenchyme. The tissue recombination studies have also demonstrated that there is a narrow developmental window for the epithelial cell fate determination in MD-derived organs. Accordingly, the developmental plasticity of epithelial cells is mostly lost in mature FRT. If the signaling that controls epithelial differentiation is disrupted at the critical developmental stage, the cell fate of MD-derived epithelial tissues will be permanently altered and can result in epithelial lesions in adult life. A disruption of signaling that maintains epithelial cell fate can also cause epithelial lesions in the FRT. In this review, the pathogenesis of cervical/vaginal adenoses and uterine squamous metaplasia is discussed as examples of such incidences.
Copyright © 2011. Published by Elsevier B.V.
Figures



References
-
- Accetta SG, Rivoire WA, Monego HI, Vettori DV, De Oliveira Freitas DM, Edelweiss MI, Capp E. Vaginal adenosis in a non-diethylstilbestrol-exposed 6-year-old patient. Gynecologic and obstetric investigation. 2001;51:271–273. - PubMed
-
- Acién P. Embryological observations on the female genital tract. Human reproduction (Oxford, England) 1992;7:437–445. - PubMed
-
- Arey LB. Developmental anatomy: a textbook and laboratory manual of embryology. W.B. Saunders; Philadelphia: 1954.
-
- Boutin EL, Battle E, Cunha GR. The response of female urogenital tract epithelia to mesenchymal inductors is restricted by the germ layer origin of the epithelium: prostatic inductions. Differentiation; research in biological diversity. 1991a;48:99–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

