Interaction of H+ with the extracellular and intracellular aspects of hMATE1
- PMID: 21613419
- PMCID: PMC3174545
- DOI: 10.1152/ajprenal.00075.2011
Interaction of H+ with the extracellular and intracellular aspects of hMATE1
Abstract
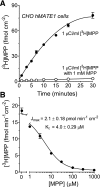
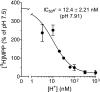
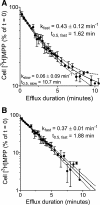
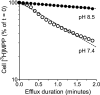
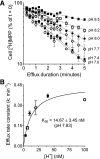
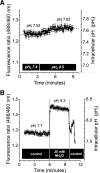
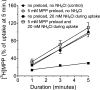
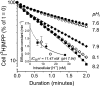
Human multidrug and toxin extrusion 1 (hMATE1, SLC47A1) is a major candidate for being the molecular identity of organic cation/proton (OC/H(+)) exchange activity in the luminal membrane of renal proximal tubules. Although physiological function of hMATE1 supports luminal OC efflux, the kinetics of hMATE1-mediated OC transport have typically been characterized through measurement of uptake, i.e., the interaction between outward-facing hMATE1 and OCs. To examine kinetics of hMATE1-mediated transport in a more physiologically relevant direction, i.e., an interaction between inward-facing hMATE1 and cytoplasmic substrates, we measured the time course of hMATE1-mediated efflux of the prototypic MATE1 substrate, [(3)H]1-methyl-4-phenylpyridinium, under a variety of intra- and extracellular pH conditions, from Chinese hamster ovary cells that stably expressed the transporter. In this study, we showed that an IC(50)/K(i) for interaction between extracellular H(+) and outward-facing hMATE1 determined from conventional uptake experiments [12.9 ± 1.23 nM (pH 7.89); n = 9] and from the efflux protocol [14.7 ± 3.45 nM (pH 7.83); n = 3] was not significantly different (P = 0.6). Furthermore, kinetics of interaction between intracellular H(+) and inward-facing hMATE1 determined using the efflux protocol revealed an IC(50) for H(+) of 11.5 nM (pH 7.91), consistent with symmetrical interactions of H(+) with the inward-facing and outward-facing aspects of hMATE1.
Figures

References
-
- Anderson CMH, Thwaites DT. Hijacking solute carriers for proton-coupled drug transport. Physiology (Bethesda) 25: 364–377, 2010 - PubMed
-
- Bednarczyk D, Mash EA, Aavula BR, Wright SH. NBD-TMA: a novel fluorescent substrate of the peritubular organic cation transporter of renal proximal tubules. Pflügers Arch 440: 184–192, 2000 - PubMed
-
- Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol 16: 871–881, 1997 - PubMed
-
- Groves CE, Evans KK, Dantzler WH, Wright SH. Peritubular organic cation transport in isolated rabbit proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 266: F450–F458, 1994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

