Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex
- PMID: 21613496
- PMCID: PMC3398699
- DOI: 10.1523/JNEUROSCI.0839-11.2011
Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex
Abstract
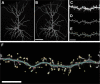
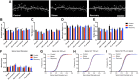
Cognitive functions that require the prefrontal cortex are highly sensitive to aging in humans, nonhuman primates, and rodents, although the neurobiological correlates of this vulnerability remain largely unknown. It has been proposed that dendritic spines represent the primary site of structural plasticity in the adult brain, and recent data have supported the hypothesis that aging is associated with alterations of dendritic spine morphology and plasticity in prefrontal cortex. However, no study to date has directly examined whether aging alters the capacity for experience-dependent spine plasticity in aging prefrontal neurons. To address this possibility, we used young, middle-aged, and aged rats in a behavioral stress paradigm known to produce spine remodeling in prefrontal cortical neurons. In young rats, stress resulted in dendritic spine loss and altered patterns of spine morphology; in contrast, spines from middle-aged and aged animals were remarkably stable and did not show evidence of remodeling. The loss of stress-induced spine plasticity observed in aging rats occurred alongside robust age-related reductions in spine density and shifts in remaining spine morphology. Together, the data presented here provide the first evidence that experience-dependent spine plasticity is altered by aging in prefrontal cortex, and support a model in which dendritic spines become progressively less plastic in the aging brain.
Figures



References
-
- Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. - PubMed
-
- Bizon JL, Helm KA, Han JS, Chun HJ, Pucilowska J, Lund PK, Gallagher M. Hypothalamic-pituitary-adrenal axis function and corticosterone receptor expression in behaviourally characterized young and aged Long–Evans rats. Eur J Neurosci. 2001;14:1739–1751. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
