Bundle-forming pilus retraction enhances enteropathogenic Escherichia coli infectivity
- PMID: 21613538
- PMCID: PMC3135470
- DOI: 10.1091/mbc.E11-01-0001
Bundle-forming pilus retraction enhances enteropathogenic Escherichia coli infectivity
Abstract
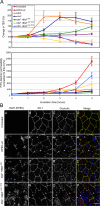
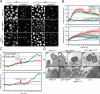
Enteropathogenic Escherichia coli (EPEC) is an important human pathogen that causes acute infantile diarrhea. The type IV bundle-forming pili (BFP) of typical EPEC strains are dynamic fibrillar organelles that can extend out and retract into the bacterium. The bfpF gene encodes for BfpF, a protein that promotes pili retraction. The BFP are involved in bacterial autoaggregation and in mediating the initial adherence of the bacterium with its host cell. Importantly, BFP retraction is implicated in virulence in experimental human infection. How pili retraction contributes to EPEC pathogenesis at the cellular level remains largely obscure, however. In this study, an effort has been made to address this question using engineered EPEC strains with induced BFP retraction capacity. We show that the retraction is important for tight-junction disruption and, to a lesser extent, actin-rich pedestal formation by promoting efficient translocation of bacterial protein effectors into the host cells. A model is proposed whereby BFP retraction permits closer apposition between the bacterial and the host cell surfaces, thus enabling timely and effective introduction of bacterial effectors into the host cell via the type III secretion apparatus. Our studies hence suggest novel insights into the involvement of pili retraction in EPEC pathogenesis.
Figures




References
-
- Allen-Vercoe E, Waddell B, Livingstone S, Deans J, DeVinney R. Enteropathogenic Escherichia coli Tir translocation and pedestal formation requires membrane cholesterol in the absence of bundle-forming pili. Cell Microbiol. 2006;8:613–624. - PubMed
-
- Bieber D, Ramer SW, Wu CY, Murray WJ, Tobe T, Fernandez R, Schoolnik GK. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

