Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size
- PMID: 21613550
- PMCID: PMC3135483
- DOI: 10.1091/mbc.E11-04-0362
Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size
Abstract
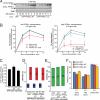
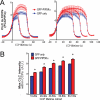
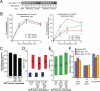
Clathrin-mediated endocytosis (CME) is the major mechanism for internalization in mammalian cells. CME initiates by recruitment of adaptors and clathrin to form clathrin-coated pits (CCPs). Nearly half of nascent CCPs abort, whereas others are stabilized by unknown mechanisms and undergo further maturation before pinching off to form clathrin-coated vesicles (CCVs). Phosphatidylinositol-(4,5)-bisphosphate (PIP(2)), the main lipid binding partner of endocytic proteins, is required for CCP assembly, but little is currently known about its contribution(s) to later events in CCV formation. Using small interfering RNA (siRNA) knockdown and overexpression, we have analyzed the effects of manipulating PIP(2) synthesis and turnover on CME by quantitative total internal reflection fluorescence microscopy and computational analysis. Phosphatidylinositol-4-phosphate-5-kinase cannot be detected within CCPs but functions in initiation and controls the rate and extent of CCP growth. In contrast, the 5'-inositol phosphatase synaptojanin 1 localizes to CCPs and controls early stabilization and maturation efficiency. Together these results suggest that the balance of PIP(2) synthesis in the bulk plasma membrane and its local turnover within CCPs control multiple stages of CCV formation.
Figures





References
-
- Bairstow SF, Ling K, Su X, Firestone AJ, Carbonara C, Anderson RA. Type Iγ661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J Biol Chem. 2006;281:20632–20642. - PubMed
-
- Barbieri MA, Heath CM, Peters EM, Wells A, Davis JN, Stahl PD. Phosphatidylinositol-4-phosphate 5-kinase-1beta is essential for epidermal growth factor receptor-mediated endocytosis. J Biol Chem. 2001;276:47212–47216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

