Calcium-dependent dynamics of cadherin interactions at cell-cell junctions
- PMID: 21613566
- PMCID: PMC3116393
- DOI: 10.1073/pnas.1019003108
Calcium-dependent dynamics of cadherin interactions at cell-cell junctions
Abstract
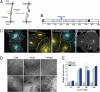
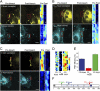
Cadherins play a key role in the dynamics of cell-cell contact formation and remodeling of junctions and tissues. Cadherin-cadherin interactions are gated by extracellular Ca(2+), which serves to rigidify the cadherin extracellular domains and promote trans junctional interactions. Here we describe the direct visualization and quantification of spatiotemporal dynamics of N-cadherin interactions across intercellular junctions in living cells using a genetically encodable FRET reporter system. Direct measurements of transjunctional cadherin interactions revealed a sudden, but partial, loss of homophilic interactions (τ = 1.17 ± 0.06 s(-1)) upon chelation of extracellular Ca(2+). A cadherin mutant with reduced adhesive activity (W2A) exhibited a faster, more substantial loss of homophilic interactions (τ = 0.86 ± 0.02 s(-1)), suggesting two types of native cadherin interactions--one that is rapidly modulated by changes in extracellular Ca(2+) and another with relatively stable adhesive activity that is Ca(2+) independent. The Ca(2+)-sensitive dynamics of cadherin interactions were transmitted to the cell interior where β-catenin translocated to N-cadherin at the junction in both cells. These data indicate that cadherins can rapidly convey dynamic information about the extracellular environment to both cells that comprise a junction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. - PubMed
-
- Tamura K, Shan WS, Hendrickson WA, Colman DR, Shapiro L. Structure-function analysis of cell adhesion by neural (N-) cadherin. Neuron. 1998;20:1153–1163. - PubMed
-
- Koch AW, Pokutta S, Lustig A, Engel J. Calcium binding and homoassociation of E-cadherin domains. Biochemistry. 1997;36:7697–7705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

