Maternal vasodilation in pregnancy: the emerging role of relaxin
- PMID: 21613576
- PMCID: PMC3154715
- DOI: 10.1152/ajpregu.00156.2011
Maternal vasodilation in pregnancy: the emerging role of relaxin
Abstract
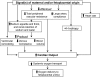
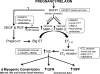
Pregnancy is a unique physiological condition of profound maternal renal and systemic vasodilation. Our goal has been to unveil the reproductive hormones mediating this remarkable vasodilatory state and the underlying molecular mechanisms. In addition to advancing our knowledge of pregnancy physiology, reaching this goal may translate into therapeutics for pregnancy pathologies such as preeclampsia and for diseases associated with vasoconstriction and arterial stiffness in nonpregnant women and men. An emerging player is the 6 kDa corpus luteal hormone relaxin, which circulates during pregnancy. Relaxin administration to rats and humans induces systemic and renal vasodilation regardless of sex, thus mimicking the pregnant condition. Immunoneutralization or elimination of the source of circulating relaxin prevents renal and systemic vasodilation in midterm pregnant rats. Infertile women who become pregnant by donor eggs (IVF with embryo transfer) lack a corpus luteum and circulating relaxin, and they show a markedly subdued gestational increase in glomerular filtration rate. These data implicate relaxin as one of the vasodilatory reproductive hormones of pregnancy. There are different molecular mechanisms underlying the so-called rapid and sustained vasodilatory actions of relaxin. The former is mediated by Gα(i/o) protein coupling to phosphatidylinositol-3 kinase/Akt (protein kinase B)-dependent phosphorylation and activation of endothelial nitric oxide synthase, the latter by vascular endothelial and placental growth factors, and increases in arterial gelatinase(s) activity. The gelatinases, in turn, hydrolyze big endothelin (ET) at a gly-leu bond to form ET(1-32), which activates the endothelial ET(B) receptor/nitric oxide vasodilatory pathway.
Figures


References
-
- Alexander BT, Miller MT, Kassab S, Novak J, Reckelhoff JF, Kruckeberg WC, Granger JP. Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats. Hypertension 33: 435–439, 1999 - PubMed
-
- August P, Lindheimer MD. Chronic hypertension and pregnancy. In: Chesley's Hypertensive Disorders in Pregnancy, edited by Lindheimer MD, Roberts JM, Cunningham GF. San Diego, CA: Academic, 2009, p. 353–368
-
- Bathgate RA, Samuel CS, Burazin TC, Gundlach AL, Tregear GW. Relaxin: new peptides, receptors and novel actions. Trends Endocrinol Metab 14: 207–213, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

