A rapid in vivo assay system for analyzing the organogenetic capacity of human kidney cells
- PMID: 21613816
- PMCID: PMC3142451
- DOI: 10.4161/org.7.2.16457
A rapid in vivo assay system for analyzing the organogenetic capacity of human kidney cells
Abstract
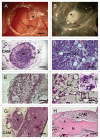
Transplantation of human kidney-derived cells is a potential therapeutic modality for promoting regeneration of diseased renal tissue. However, assays that determine the ability of candidate populations for renal cell therapy to undergo appropriate differentiation and morphogenesis are limited. We report here a rapid and humane assay for characterizing tubulogenic potency utilizing the well-established chorioallantoic membrane CAM) of the chick embryo. Adult human kidney-derived cells expanded in monolayer were suspended in Matrigel and grafted onto the CAM. After a week, grafts were assessed histologically. Strikingly, many of the renal cells self-organized into tubular structures. Host blood vessels penetrated and presumably fed the grafts. Immuno- and histochemical staining revealed that tubular structures were epithelial, but not blood vessels. Some of the cells both within and outside the tubules were dividing. Analysis for markers of proximal and distal renal tubules revealed that grafts contained individual cells of a proximal tubular phenotype and many tubules of distal tubule character. Our results demonstrate that the chick CAM is a useful xenograft system for screening for differentiation and morphogenesis in cells with potential use in renal regenerative medicine.
Figures

References
-
- Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 2003;17:1159–1161. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
