Catalysis for fluorination and trifluoromethylation
- PMID: 21614074
- PMCID: PMC3119199
- DOI: 10.1038/nature10108
Catalysis for fluorination and trifluoromethylation
Abstract
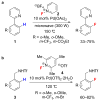
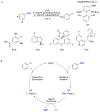
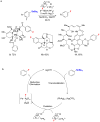
Recent advances in catalysis have made the incorporation of fluorine into complex organic molecules easier than ever before, but selective, general and practical fluorination reactions remain sought after. Fluorination of molecules often imparts desirable properties, such as metabolic and thermal stability, and fluorinated molecules are therefore frequently used as pharmaceuticals or materials. But the formation of carbon-fluorine bonds in complex molecules is a significant challenge. Here we discuss reactions to make organofluorides that have emerged within the past few years and which exemplify how to overcome some of the intricate challenges associated with fluorination.
Figures


References
-
- Müller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: Looking beyond intuition. Science. 2007;317:1881–1886. - PubMed
-
- Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem Soc Rev. 2008;37:320–330. - PubMed
-
- Jeschke P. The unique role of fluorine in the design of active ingredients for modern crop production. ChemBioChem. 2004;5:570–589. - PubMed
-
- Hung MH, Farnham WB, Feiring AE, Rozen S. Functional Fluoromonomers and Fluoropolymers. In: Hougham G, Cassidy PE, Johns K, Davidson T, editors. Fluoropolymers: Synthesis. Vol. 1. Plenum Publishing Co; 1999. pp. 51–66.
-
- Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chem Rev. 2008;108:1501–1516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

