Reciprocal influence of the p53 and the hypoxic pathways
- PMID: 21614094
- PMCID: PMC3122125
- DOI: 10.1038/cddis.2011.48
Reciprocal influence of the p53 and the hypoxic pathways
Abstract
When cells sense a decrease in oxygen availability (hypoxia), they develop adaptive responses in order to sustain this condition and survive. If hypoxia lasts too long or is too severe, the cells eventually die. Hypoxia is also known to modulate the p53 pathway, in a manner dependent or not of HIF-1 (hypoxia-inducible factor-1), the main transcription factor activated by hypoxia. The p53 protein is a transcription factor, which is rapidly stabilised by cellular stresses and which has a major role in the cell responses to these stresses. The aim of this review is to compile what has been reported until now about the interconnection between these two important pathways. Indeed, according to the cell line, the severity and the duration of hypoxia, oxygen deficiency influences very differently p53 protein level and activity. Conversely, p53 is also described to affect HIF-1α stability, one of the two subunits of HIF-1, and HIF-1 activity. The direct and indirect interactions between HIF-1α and p53 are described as well as the involvement in this complex network of their respective ubiquitin ligases von Hippel Lindau protein and murine double minute 2. Finally, the synergistic or antagonistic effects of p53 and HIF-1 on some important cellular pathways are discussed.
Figures
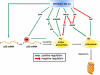
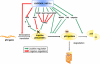
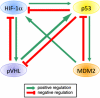

References
-
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. - PubMed
-
- Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1:233–240. - PubMed
-
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. - PubMed
-
- Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

