Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes
- PMID: 21614186
- PMCID: PMC3100834
- DOI: 10.3390/ijms11124973
Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes
Abstract
Extractability, extractable protein compositions, technological-functional properties of pea (Pisum sativum) proteins from six genotypes grown in Serbia were investigated. Also, the relationship between these characteristics was presented. Investigated genotypes showed significant differences in storage protein content, composition and extractability. The ratio of vicilin:legumin concentrations, as well as the ratio of vicilin + convicilin: Legumin concentrations were positively correlated with extractability. Our data suggest that the higher level of vicilin and/or a lower level of legumin have a positive influence on protein extractability. The emulsion activity index (EAI) was strongly and positively correlated with the solubility, while no significant correlation was found between emulsion stability (ESI) and solubility, nor between foaming properties and solubility. No association was evident between ESI and EAI. A moderate positive correlation between emulsion stability and foam capacity was observed. Proteins from the investigated genotypes expressed significantly different emulsifying properties and foam capacity at different pH values, whereas low foam stability was detected. It appears that genotype has considerable influence on content, composition and technological-functional properties of pea bean proteins. This fact can be very useful for food scientists in efforts to improve the quality of peas and pea protein products.
Keywords: emulsifying; extractability; foaming; pea proteins.
Figures
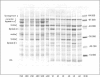
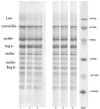





References
-
- Roy F, Boye JI, Simpson BK. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010;43:432–442.
-
- Gueguen JCM, Barbot J, Schaeffer F. Dissociation and aggregation of pea legumin induced by pH and ionic strength. J. Sci. Food Agric. 1988;53:167–182.
-
- Heng L, van Koningsveld GA, Gruppen H, van Boekel MAJS, Vincken JP, Roozen JP, Voragen AGJ. Protein-flavour interactions in relation to development of novel protein foods. Trends Food Sci. Technol. 2004;15:217–224.
-
- Griga M, Horáček J, Klenotičová H. Protein patterns associated with Pisum sativum somatic embryogenESIs. Biol. Plantarum. 2007;51:201–211.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

