Protease-resistant peptide ligands from a knottin scaffold library
- PMID: 21615106
- PMCID: PMC3158827
- DOI: 10.1021/cb200039s
Protease-resistant peptide ligands from a knottin scaffold library
Abstract
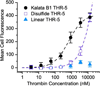
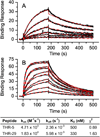
Peptides within the knottin family have been shown to possess inherent stability, making them attractive scaffolds for the development of therapeutic and diagnostic agents. Given its remarkable stability to proteases, the cyclic peptide kalata B1 was employed as a scaffold to create a large knottin library displayed on the surface of E. coli. A library exceeding 10(9) variants was constructed by randomizing seven amino acids within a loop of the kalata B1 scaffold and screened using fluorescence-activated cell sorting to identify peptide ligands specific for the active site of human thrombin. Refolded thrombin binders exhibited high nanomolar affinities in solution and slow dissociation rates and were able to inhibit thrombin's enzymatic activity. Importantly, 80% of a knottin-based thrombin inhibitor remained intact after a 2 h incubation both with trypsin and with chymotrypsin, demonstrating that modifying the kalata B1 sequence did not compromise its stability properties. In addition, the knottin variant mediated 20-fold enhanced affinity for thrombin, when compared to the same seven residue binding epitope constrained by a single disulfide bond. Our results indicate that peptide libraries derived from the kalata B1 scaffold can yield high-affinity protein ligands that retain the remarkable protease resistance associated with the parent scaffold. More generally, this strategy may prove useful in the development of stable peptide ligands suitable for in vivo applications.
Figures



References
-
- Pichereau C, Allary C. Therapeutic peptides under the spotlight. Eur. Biopharm. Rev. 2005:88–91.
-
- Sato AK, Viswanathan M, Kent RB, Wood CR. Therapeutic peptides: technological advances driving peptides into development. Curr. Opin. Biotechnol. 2006;17:638–642. - PubMed
-
- Werle M, Bernkop-Schnürch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30:351–367. - PubMed
-
- McGregor DP. Discovering and improving novel peptide therapeutics. Curr. Opin. Pharm. 2008;8:616–619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

