Identification of phenylalanine 3-hydroxylase for meta-tyrosine biosynthesis
- PMID: 21615132
- PMCID: PMC3115494
- DOI: 10.1021/bi200733c
Identification of phenylalanine 3-hydroxylase for meta-tyrosine biosynthesis
Abstract
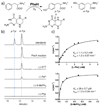
Phenylalanine hydroxylase (PheH) is an iron(II)-dependent enzyme that catalyzes the hydroxylation of aromatic amino acid l-phenylalanine (L-Phe) to l-tyrosine (L-Tyr). The enzymatic modification has been demonstrated to be highly regiospecific, forming proteinogenic para-Tyr (p-Tyr) exclusively. Here we biochemically characterized the first example of a phenylalanine 3-hydroxylase (Phe3H) that catalyzes the synthesis of meta-Tyr (m-Tyr) from Phe. Subsequent mutagenesis studies revealed that two residues in the active site of Phe3H (Cys187 and Thr202) contribute to C-3 rather than C-4 hydroxylation of the phenyl ring. This work sets the stage for the mechanistic and structural study of regiospecific control of the substrate hydroxylation by PheH.
Figures
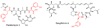

References
-
- Flatmark T, Stevens RC. Structural insight into the aromatic amino acid hydroxylases and their disease-related mutant forms. Chem. Rev. 1999;99:2137–2160. - PubMed
-
- Fitzpatrick PF. Kinetic isotope effects on hydroxylation of ring-deuterated phenylalanines by tyrosine hydroxylase provide evidence against partitioning of an arene oxide intermediate. J. Am. Chem. Soc. 1994;116:1133–1134.
-
- Erlandsen H, Bjorgo E, Flatmark T, Stevens RC. Crystal structure and site-specific mutagenesis of pterin-bound human phenylalanine hydroxylase. Biochemistry. 2000;39:2208–2217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

