Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles
- PMID: 21615170
- PMCID: PMC3144304
- DOI: 10.1021/nn201397z
Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles
Abstract
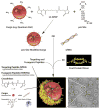
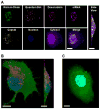
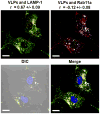
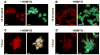
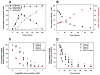
Virus-like particles (VLPs) of bacteriophage MS2 possess numerous features that make them well-suited for use in targeted delivery of therapeutic and imaging agents. MS2 VLPs can be rapidly produced in large quantities using in vivo or in vitro synthesis techniques. Their capsids can be modified in precise locations via genetic insertion or chemical conjugation, facilitating the multivalent display of targeting ligands. MS2 VLPs also self-assemble in the presence of nucleic acids to specifically encapsidate siRNA and RNA-modified cargos. Here we report the use of MS2 VLPs to selectively deliver nanoparticles, chemotherapeutic drugs, siRNA cocktails, and protein toxins to human hepatocellular carcinoma (HCC). MS2 VLPs modified with a peptide (SP94) that binds HCC exhibit a 10(4)-fold higher avidity for HCC than for hepatocytes, endothelial cells, monocytes, or lymphocytes and can deliver high concentrations of encapsidated cargo to the cytosol of HCC cells. SP94-targeted VLPs loaded with doxorubicin, cisplatin, and 5-fluorouracil selectively kill the HCC cell line, Hep3B, at drug concentrations <1 nM, while SP94-targeted VLPs that encapsidate a siRNA cocktail, which silences expression of cyclin family members, induce growth arrest and apoptosis of Hep3B at siRNA concentrations <150 pM. Impressively, MS2 VLPs, when loaded with ricin toxin A-chain (RTA) and modified to codisplay the SP94 targeting peptide and a histidine-rich fusogenic peptide (H5WYG) that promotes endosomal escape, kill virtually the entire population of Hep3B cells at an RTA concentration of 100 fM without affecting the viability of control cells. Our results demonstrate that MS2 VLPs, because of their tolerance of multivalent peptide display and their ability to specifically encapsidate a variety of chemically disparate cargos, induce selective cytotoxicity of cancer in vitro and represent a significant improvement in the characteristics of VLP-based delivery systems.
Figures

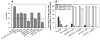
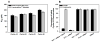


References
-
- Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R. Nanocarriers as an Emerging Platform for Cancer Ttherapy. Nature Nanotech. 2007;2:751–760. - PubMed
-
- Carrico ZM, Romanini DW, Mehl RA, Francis MB. Oxidative Coupling of Peptides to a Virus Capsid Containing Unnatural Amino Acids. Chem Commun. 2008:1207–1207. - PubMed
-
- Hooker JM, Datta A, Botta M, Raymond KN, Francis MB. Magnetic Resonance Contrast Agents from Viral Capsid Shells: A Comparison of Exterior and Interior Cargo Strategies. Nano Lett. 2007;7:2207–2210. - PubMed
-
- Kovacs EW, Hooker JM, Romanini DW, Holder PG, Berry KE, Francis MB. Dual-Surface-Modified Bacteriophage MS2 as an Ideal Scaffold for a Viral Capsid-Based Drug Delivery System. Bioconjugate Chem. 2007;18:1140–1147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

