The FbaB-type fibronectin-binding protein of Streptococcus pyogenes promotes specific invasion into endothelial cells
- PMID: 21615663
- PMCID: PMC4754676
- DOI: 10.1111/j.1462-5822.2011.01610.x
The FbaB-type fibronectin-binding protein of Streptococcus pyogenes promotes specific invasion into endothelial cells
Abstract
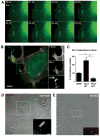
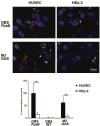
Invasive serotype M3 Streptococcus pyogenes are among the most frequently isolated organisms from patients suffering from invasive streptococcal disease and have the potential to invade primary human endothelial cells (EC) via a rapid and efficient mechanism. FbaB protein, the fibronectin-binding protein expressed by M3 S. pyogenes, was herein identified as a potent invasin for EC. By combining heterologous gene expression with allelic replacement, we demonstrate that FbaB is essential and sufficient to trigger EC invasion via a Rac1-dependent phagocytosis-like uptake. FbaB-mediated uptake follows the classical endocytic pathway with lysosomal destination. FbaB is demonstrated to be a streptococcal invasin exhibiting EC tropism. FbaB thus initiates a process that may contribute to the deep tissue tropism and spread of invasive S. pyogenes isolates into the vascular EC lining.
© 2011 Blackwell Publishing Ltd.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

