Marking the tempo for myogenesis: Pax7 and the regulation of muscle stem cell fate decisions
- PMID: 21615681
- PMCID: PMC4365881
- DOI: 10.1111/j.1582-4934.2011.01348.x
Marking the tempo for myogenesis: Pax7 and the regulation of muscle stem cell fate decisions
Abstract
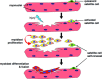
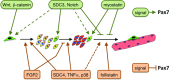
Post-natal growth and regeneration of skeletal muscle is highly dependent on a population of resident myogenic precursors known as satellite cells. Transcription factors from the Pax gene family, Pax3 and Pax7, are critical for satellite cell biogenesis, survival and potentially self-renewal; however, the underlying molecular mechanisms remain unsolved. This is particularly true in the case of Pax7, which appears to regulate myogenesis at multiple levels. Accordingly, recent data have highlighted the importance of a functional relationship between Pax7 and the MyoD family of muscle regulatory transcription factors during normal muscle formation and disease. Here we will critically review key findings suggesting that Pax7 may play a dual role by promoting resident muscle progenitors to commit to the skeletal muscle lineage while preventing terminal differentiation, thus keeping muscle progenitors poised to differentiate upon environmental cues. In addition, potential regulatory mechanisms for the control of Pax7 activity will be proposed.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures


References
-
- Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14:82–91. - PubMed
-
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–51. - PubMed
-
- Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol. 1994;123:213–57. - PubMed
-
- Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J Exp Zool. 1978;206:451–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

