Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury
- PMID: 21615744
- PMCID: PMC3179800
- DOI: 10.1111/j.1537-2995.2011.03186.x
Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury
Abstract
Background: Lipids accumulate during the storage of red blood cells (RBCs), prime neutrophils (PMNs), and have been implicated in transfusion-related acute lung injury (TRALI). These lipids are composed of two classes: nonpolar lipids and lysophosphatidylcholines based on their retention time on separation by high-pressure liquid chromatography. Prestorage leukoreduction significantly decreases white blood cell and platelet contamination of RBCs; therefore, it is hypothesized that prestorage leukoreduction changes the classes of lipids that accumulate during storage, and these lipids prime PMNs and induce acute lung injury (ALI) as the second event in a two-event in vivo model.
Study design and methods: RBC units were divided: 50% was leukoreduced (LR-RBCs), stored, and sampled on Day 1 and at the end of storage, Day 42. Priming activity was evaluated on isolated PMNs, and the purified lipids from Day 1 or Day 42 were used as the second event in the in vivo model.
Results: The plasma and lipids from RBCs and LR-RBCs primed PMNs, and the LR-RBC activity decreased with longer storage. Unlike RBCs, nonpolar lipids comprised the PMN-priming activity from stored LR-RBCs. Mass spectroscopy identified these lipids as arachidonic acid and 5-, 12-, and 15-hydroxyeicsotetranoic acid. At concentrations from Day 42, but not Day 1, three of four of these lipids individually, and the mixture, primed PMNs. The mixture also caused ALI as the second event in a two-event model of TRALI.
Conclusion: We conclude that the nonpolar lipids that accumulate during LR-RBC storage may represent the agents responsible for antibody-negative TRALI.
© 2011 American Association of Blood Banks.
Figures
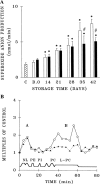
 ) and Day 1 plasma from both LR-RBCs and RBC-priming activity was augmented on Day 14 for both unmodified RBCs (□) and LR-RBCs (■) and steadily increased in the plasma from unmodified RBCs reaching a relative maximum on Day 42. In contrast the plasma-priming activity from LR-RBCs appeared to plateau on Day 28 of storage and was significantly decreased compared to the plasma-priming activity from unmodified RBCs on Days 35 and Day 42 of storage. *p < 0.05 compared to buffer or Day 7 plasma primed PMNs; #p < 0.05 compared to the nonmodified plasma from Day 35 and Day 42. (B) Separation of the lipids from RBCs that cause priming activity in PMNs. The lipid peaks that cause priming activity in PMNs (multiple of albumin-treated controls) are shown as a function of the retention time of lipid separation by normal-phase HPLC. The unmodified RBC plasma (◯) demonstrated two peaks of PMN-priming activity, the first at the retention time of nonpolar lipids and the second a broad peak of activity known to contain lyso-PCs. In contrast separation of the plasma lipid peaks from Day 42 LR-RBCs (▲) yielded only a single lipid peak of involved in PMN-priming activity at the retention time of nonpolar lipids. The data are expressed as fold concentration of the mean from three separate experiments using disparate RBCs from different donors. D = day; NL = nonpolar lipids; PE = phosphatidylethanolamine; PI = phosphatidylinositol; PC = phosphatidylcholine; L-PC = lyso-PC.
) and Day 1 plasma from both LR-RBCs and RBC-priming activity was augmented on Day 14 for both unmodified RBCs (□) and LR-RBCs (■) and steadily increased in the plasma from unmodified RBCs reaching a relative maximum on Day 42. In contrast the plasma-priming activity from LR-RBCs appeared to plateau on Day 28 of storage and was significantly decreased compared to the plasma-priming activity from unmodified RBCs on Days 35 and Day 42 of storage. *p < 0.05 compared to buffer or Day 7 plasma primed PMNs; #p < 0.05 compared to the nonmodified plasma from Day 35 and Day 42. (B) Separation of the lipids from RBCs that cause priming activity in PMNs. The lipid peaks that cause priming activity in PMNs (multiple of albumin-treated controls) are shown as a function of the retention time of lipid separation by normal-phase HPLC. The unmodified RBC plasma (◯) demonstrated two peaks of PMN-priming activity, the first at the retention time of nonpolar lipids and the second a broad peak of activity known to contain lyso-PCs. In contrast separation of the plasma lipid peaks from Day 42 LR-RBCs (▲) yielded only a single lipid peak of involved in PMN-priming activity at the retention time of nonpolar lipids. The data are expressed as fold concentration of the mean from three separate experiments using disparate RBCs from different donors. D = day; NL = nonpolar lipids; PE = phosphatidylethanolamine; PI = phosphatidylinositol; PC = phosphatidylcholine; L-PC = lyso-PC.
 ) or Day 42 (▧) of LR-RBC storage are shown. *p < 0.05 versus the controls, both buffer and albumin treated, and the concentrations of the individual lipid moieties or mixture on Day 1 of storage. Each bar represents a sample size of seven. AA = arachidonic acid.
) or Day 42 (▧) of LR-RBC storage are shown. *p < 0.05 versus the controls, both buffer and albumin treated, and the concentrations of the individual lipid moieties or mixture on Day 1 of storage. Each bar represents a sample size of seven. AA = arachidonic acid.
 ) NS; (■) LPS.
) NS; (■) LPS.Comment in
-
Red blood cells, transfusion-related acute lung injury, and lipids: a role for liporeduction?Transfusion. 2011 Dec;51(12):2524-6. doi: 10.1111/j.1537-2995.2011.03307.x. Transfusion. 2011. PMID: 22150614 Free PMC article. No abstract available.
References
-
- Holness L, Knippen MA, Simmons L, Lachenbruch PA. Fatalities caused by TRALI. Transfus Med Rev. 2004;18:184–8. - PubMed
-
- Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–62. - PMC - PubMed
-
- Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–7. - PubMed
-
- Silliman CC, Boshkov LK, Mehdizadehkashi Z, Elzi DJ, Dickey WO, Podlosky L, Clarke G, Ambruso DR. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

