Function of the asparagine 74 residue of the inhibitory γ-subunit of retinal rod cGMP-phophodiesterase (PDE) in vivo
- PMID: 21616145
- PMCID: PMC3148328
- DOI: 10.1016/j.cellsig.2011.05.007
Function of the asparagine 74 residue of the inhibitory γ-subunit of retinal rod cGMP-phophodiesterase (PDE) in vivo
Abstract
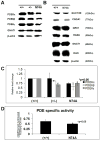
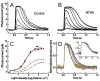
The inhibitory subunit of rod cyclic guanosine monophosphate (cGMP) phosphodiesterase, PDE6γ, is a major component of rod transduction and is required to support photoreceptor integrity. The N74A allele of PDE6γ has previously been shown in experiments carried out in vitro to reduce the regulatory inhibition on the PDE6 catalytic core subunits, PDE6αβ. This should, in intact rods, lead to an increase in basal (dark) PDE6 activity producing a state equivalent to light adaptation in the rods and we have examined this possibility using ERG and suction-electrode measurements. The murine opsin promoter was used to drive the expression of a mutant N74A and a wild-type PDE6γ control transgene in the photoreceptors of +/Pde6g(tm1) mice. This transgenic line was crossed with Pde6g(tm1)/Pde6g(tm1) mice to generate animals able to synthesize only the transgenic mutant PDE6γ. We find that the N74A mutation did not produce a significant decrease in circulating current, a decrease in sensitivity or affect the kinetics of the light response, all hallmarks of the light-adapted state. In an in vitro assay of the PDE purified from the N74A transgenic mice and control mice we could find no increase in basal activity of the mutant PDE6. Both the results from the physiology and the biochemistry experiments are consistent with the interpretation that the mutation causes a much milder phenotype in vivo than was predicted from observations made using a cell-free assay system. The in vivo regulation of PDE6γ on PDE6αβ may be more dynamic and context-dependent than was replicated in vitro.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


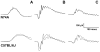
References
-
- Granovsky AE, Arte[Cowan, #1466]myev NO. Biochemistry. 2001;40(44):13209–13215. - PubMed
-
- Tsang SH, Gouras P. In: Duane’s Clinical Opthalmology. Tasman W, Jaeger EA, editors. Chapter 2 Philadelphia: J.B Lippincott; 1996.
-
- Farber DB, Tsang SH. Front Biosci. 2003;8:S666–675. - PubMed
-
- Qin N, Baehr W. J Biol Chem. 1994;269:3265–3271. - PubMed
-
- Muradov H, Boyd KK, Artemyev NO. Vision Res. 2004;44(21):2437–2444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

