Early alveolar epithelial dysfunction promotes lung inflammation in a mouse model of Hermansky-Pudlak syndrome
- PMID: 21616998
- PMCID: PMC3175543
- DOI: 10.1164/rccm.201011-1882OC
Early alveolar epithelial dysfunction promotes lung inflammation in a mouse model of Hermansky-Pudlak syndrome
Abstract
Rationale: The pulmonary phenotype of Hermansky-Pudlak syndrome (HPS) in adults includes foamy alveolar type 2 cells, inflammation, and lung remodeling, but there is no information about ontogeny or early disease mediators.
Objectives: To establish the ontogeny of HPS lung disease in an animal model, examine disease mediators, and relate them to patients with HPS1.
Methods: Mice with mutations in both HPS1/pale ear and HPS2/AP3B1/pearl (EPPE mice) were studied longitudinally. Total lung homogenate, lung tissue sections, and bronchoalveolar lavage (BAL) were examined for phospholipid, collagen, histology, cell counts, chemokines, surfactant protein D (SP-D), and S-nitrosylated SP-D. Isolated alveolar epithelial cells were examined for expression of inflammatory mediators, and chemotaxis assays were used to assess their importance. Pulmonary function test results and BAL from patients with HPS1 and normal volunteers were examined for clinical correlation.
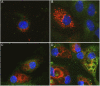
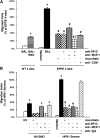
Measurements and main results: EPPE mice develop increased total lung phospholipid, followed by a macrophage-predominant pulmonary inflammation, and lung remodeling including fibrosis. BAL fluid from EPPE animals exhibited early accumulation of both SP-D and S-nitrosylated SP-D. BAL fluid from patients with HPS1 exhibited similar changes in SP-D that correlated inversely with pulmonary function. Alveolar epithelial cells demonstrated expression of both monocyte chemotactic protein (MCP)-1 and inducible nitric oxide synthase in juvenile EPPE mice. Last, BAL from EPPE mice and patients with HPS1 enhanced migration of RAW267.4 cells, which was attenuated by immunodepletion of SP-D and MCP-1.
Conclusions: Inflammation is initiated from the abnormal alveolar epithelial cells in HPS, and S-nitrosylated SP-D plays a significant role in amplifying pulmonary inflammation.
Figures






References
-
- Gochuico BR, Huizing M, Golas G, Gahl WA. Interstitial lung disease in Hermansky-Pudlak syndrome-2. Am J Respir Crit Care Med 2010;181:A6018
-
- Nakatani Y, Nakamura N, Sano J, Inayama Y, Kawano N, Yamanaka S, Miyagi Y, Nagashima Y, Ohbayashi C, Mizushima M, et al. Interstitial pneumonia in Hermansky-Pudlak syndrome: significance of florid foamy swelling/degeneration (giant lamellar body degeneration) of type-2 pneumocytes. Virchows Arch 2000;437:304–313 - PubMed
-
- Lyerla TA, Rusiniak ME, Borchers M, Jahreis G, Tan J, Ohtake P, Novak EK, Swank RT. Aberrant lung structure, composition, and function in a murine model of Hermansky-Pudlak syndrome. Am J Physiol Lung Cell Mol Physiol 2003;285:L643–L653 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL086621/HL/NHLBI NIH HHS/United States
- R01 HL064520/HL/NHLBI NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
- HL059959/HL/NHLBI NIH HHS/United States
- R01 HL059959/HL/NHLBI NIH HHS/United States
- P01 HL019737/HL/NHLBI NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- R56 HL059959/HL/NHLBI NIH HHS/United States
- HL064520/HL/NHLBI NIH HHS/United States
- R01 HL086621/HL/NHLBI NIH HHS/United States
- HL019737/HL/NHLBI NIH HHS/United States
- ES013508/ES/NIEHS NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

